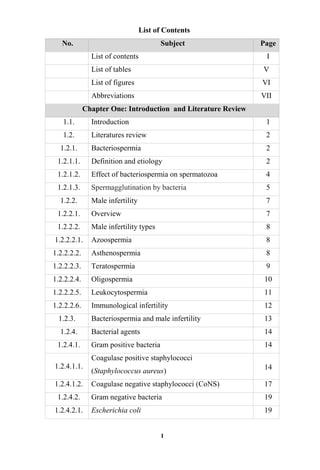
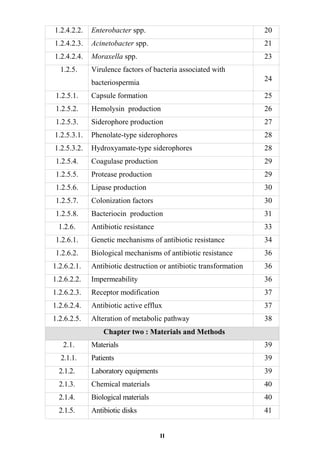
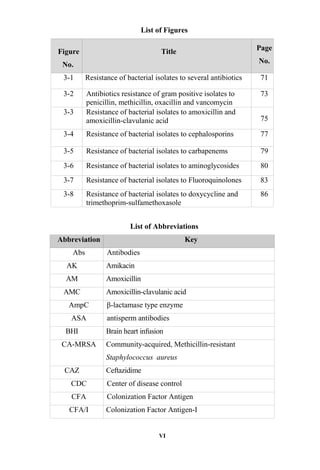
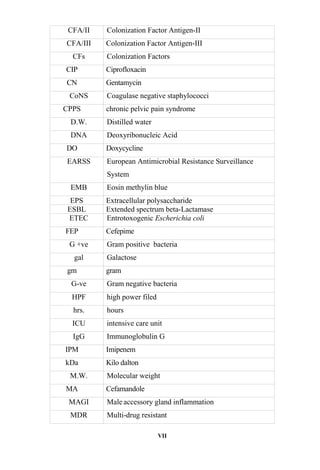
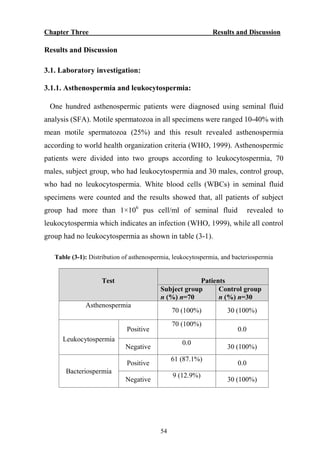
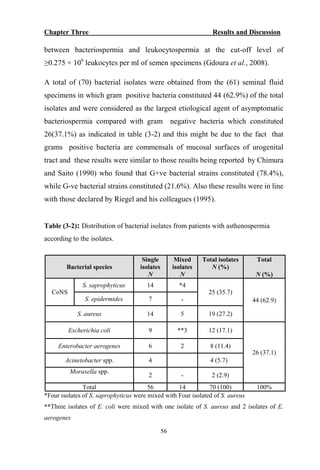
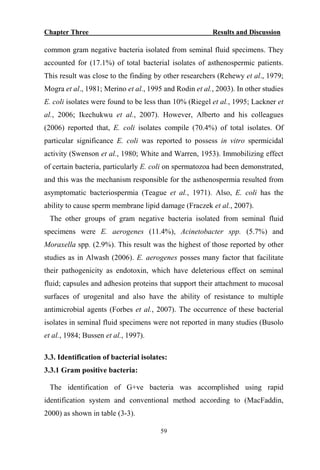
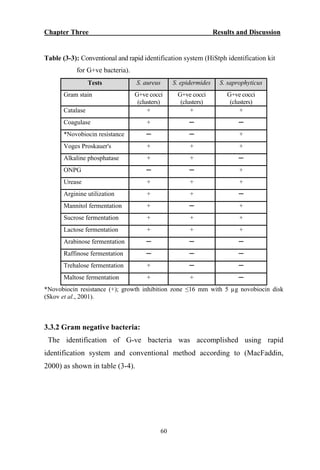
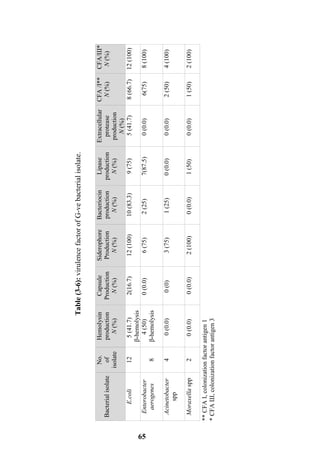
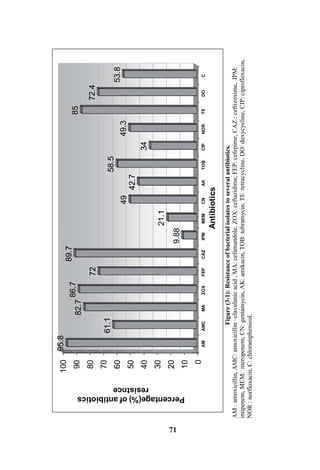
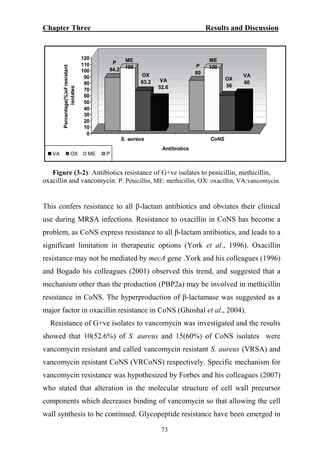
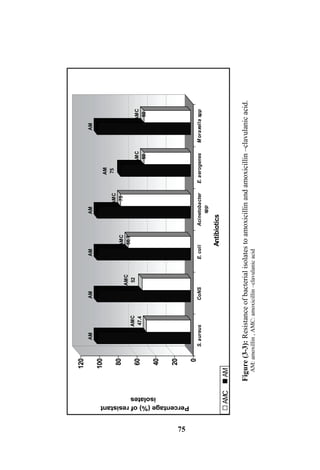
The document discusses bacteriospermia, a condition characterized by the presence of bacteria in seminal fluid, which is linked to male infertility, particularly asthenospermia. It includes an extensive literature review, highlighting the impact of bacterial infection on sperm function and various male infertility types. The work aims to investigate the relationship between bacteriospermia and leukocytospermia in infertile men and to identify common uropathogenic bacterial species along with their virulence factors and antibiotic resistance patterns.





















































































































![References
128
Rahal, J. (2006). Novel antibiotic combinations against infections with almost
completely resistant Pseudomonas aeruginosa and Acinetobacter
species. Clin. Infect. Dis., 43(2):95–99.
Rajasekaran, M., Hellstrom W. J., Naz R. K., and Sikka S.C.(1995). Oxidative
stress and interleukins in seminal plasma during
leukocytospermia. Fertil. Steril., 64:166-171.
Rajesh, B. and Rutten I. (2004). Essential medical microbiology. 3rd ed. New
Delhi–Indian.
Raymond, K. N., Dertz E. A. and Kim S. S. (2003). Enterobactin: an archetype
for microbial iron transport. Proc. Natl. Acad. Sci. USA. , 100:
3584-3588.
Raz, R., Colodner R. and Kunin C. M. (2005). Who are you -Staphylococcus
saprophyticus? CID [serial online]; 40:896-898.
Rehewy, M. S. E., Hafez E. S. E., Thomas A. and Brown W. J. (1979).
Aerobic and anaerobic bacterial flora in semen from fertile and
infertile groups of men. Arch. Andrology, (2): 238-263.
Reichart, M., Kahane I. and Bartoov B. (2000).In Vivo and In Vitro
Impairment of Human and Ram Sperm Nuclear Chromatin
Integrity by Sexually Transmitted Ureaplasma urealyticum.
Infect. Biol .Reprod. 63:1041-1048.
Reichart, M., Kahane I. and Bartoov B. (2001). Dual energy metabolism-
dependent effect of Ureaplasma urealyticum infection on sperm
activity. J. Androl., 22:404-412.
Riegel, P., Ruimy R., Briel D., Prevost G., Jehl F., Bimet F., Christen R. and
Monteil H. (1995). Corynebacterium seminali sp. Nov., new
species associated with genital infection in male patients. J.
Clinic. Microbiol., 33(9) .p. 2244-2249.](https://image.slidesharecdn.com/bacteriospermia-978-3-659-34491-6-160718201731/85/Bacteriospermia-145-320.jpg)















