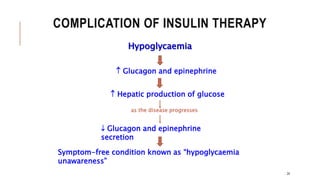
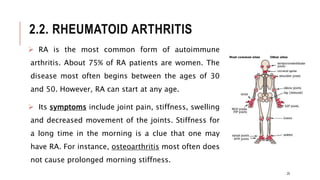
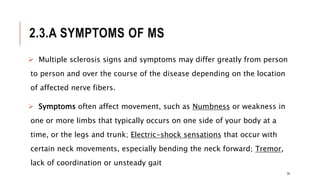
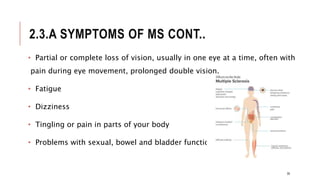
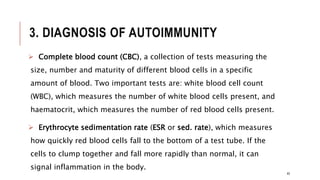
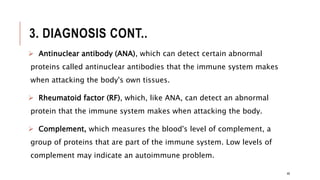
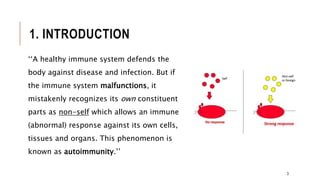
This document provides an overview of autoimmune diseases. It begins by defining autoimmunity as the immune system mistakenly attacking the body's own cells and tissues. Some common autoimmune diseases are then discussed in more detail, including type 1 diabetes, rheumatoid arthritis, and multiple sclerosis. The causes of autoimmunity are largely unknown but may involve genetic and environmental factors. Symptoms vary depending on the specific disease. Diagnosis and treatment options are also summarized for some of the major autoimmune diseases.










![2.1.B. DIAGNOSIS OF T1DM
The diagnosis is confirmed by a glycosylated haemoglobin
concentration ≥ 6.5 mg/dl (normal < 5.7). [Note: The rate of formation
of HbA1c is proportional to the average blood glucose concentration
over the previous 3 months.]
The diagnosis can also be confirmed by a Fasting Blood Glucose test
(FBG) ≥ 126 mg/dl (normal is 70–99). [Note: A FBG of 100–125 mg/dl is
categorized as an impaired FBG.] Fasting is defined as no caloric intake
for at least 8 hours. The diagnosis can also be made on the basis of a
non-fasting (random) blood glucose level greater than 200 mg/dl in an
individual with symptoms of hyperglycaemia.
15](https://image.slidesharecdn.com/autoimmunedisorders-200120153121/85/Autoimmune-Disorders-15-320.jpg)
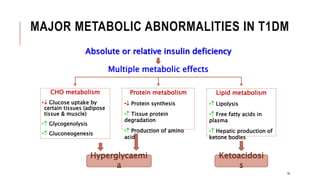
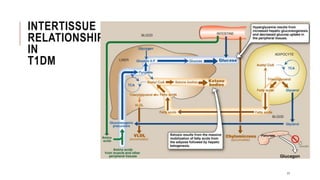
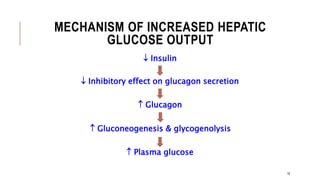

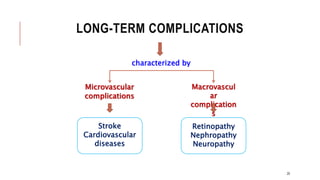
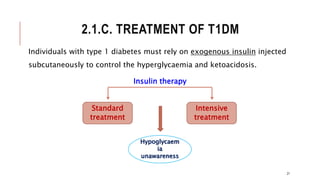
![2.1.C. TREATMENT OF T1DM CONT..
I. Standard insulin therapy:
Standard treatment typically consists of one or two daily injections of
recombinant human insulin.
Mean blood glucose levels obtained are typically in the 225–275 mg/dl
range, with a haemoglobin A1C (HbA1c) level of 8–9% of the total
haemoglobin. [Note: Normal mean blood glucose is approximately 100
mg/dl, and HbA1c is 6% or less.]
22](https://image.slidesharecdn.com/autoimmunedisorders-200120153121/85/Autoimmune-Disorders-22-320.jpg)
![2.1.C. TREATMENT OF T1DM CONT..
II. Intensive insulin therapy:
Intensive treatment seeks to more closely normalize blood glucose
through more frequent monitoring and subsequent injections of insulin,
typically three or more times a day.
Mean blood glucose levels of 150 mg/dl can be achieved, with HbA1c
approximately 7% of the total haemoglobin. [Note: Normal mean blood
glucose is approximately 100 mg/dl, and HbA1c is 6% or less.]
23](https://image.slidesharecdn.com/autoimmunedisorders-200120153121/85/Autoimmune-Disorders-23-320.jpg)