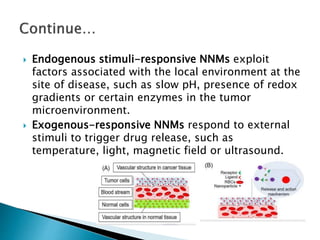
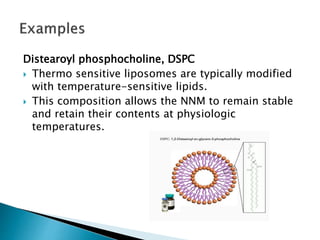
Nano-medicine, which utilizes nano-particles for targeted drug delivery, shows promise in enhancing disease prevention, diagnosis, and treatment, particularly in cancers and inflammatory diseases. Key mechanisms include solubilization, passive and active targeting, and triggered release, with an emphasis on overcoming biological barriers for effective therapeutic use. Challenges in clinical development encompass biological issues, manufacturing complexities, regulatory hurdles, and the need for robust preclinical evaluation to mitigate investment risks.