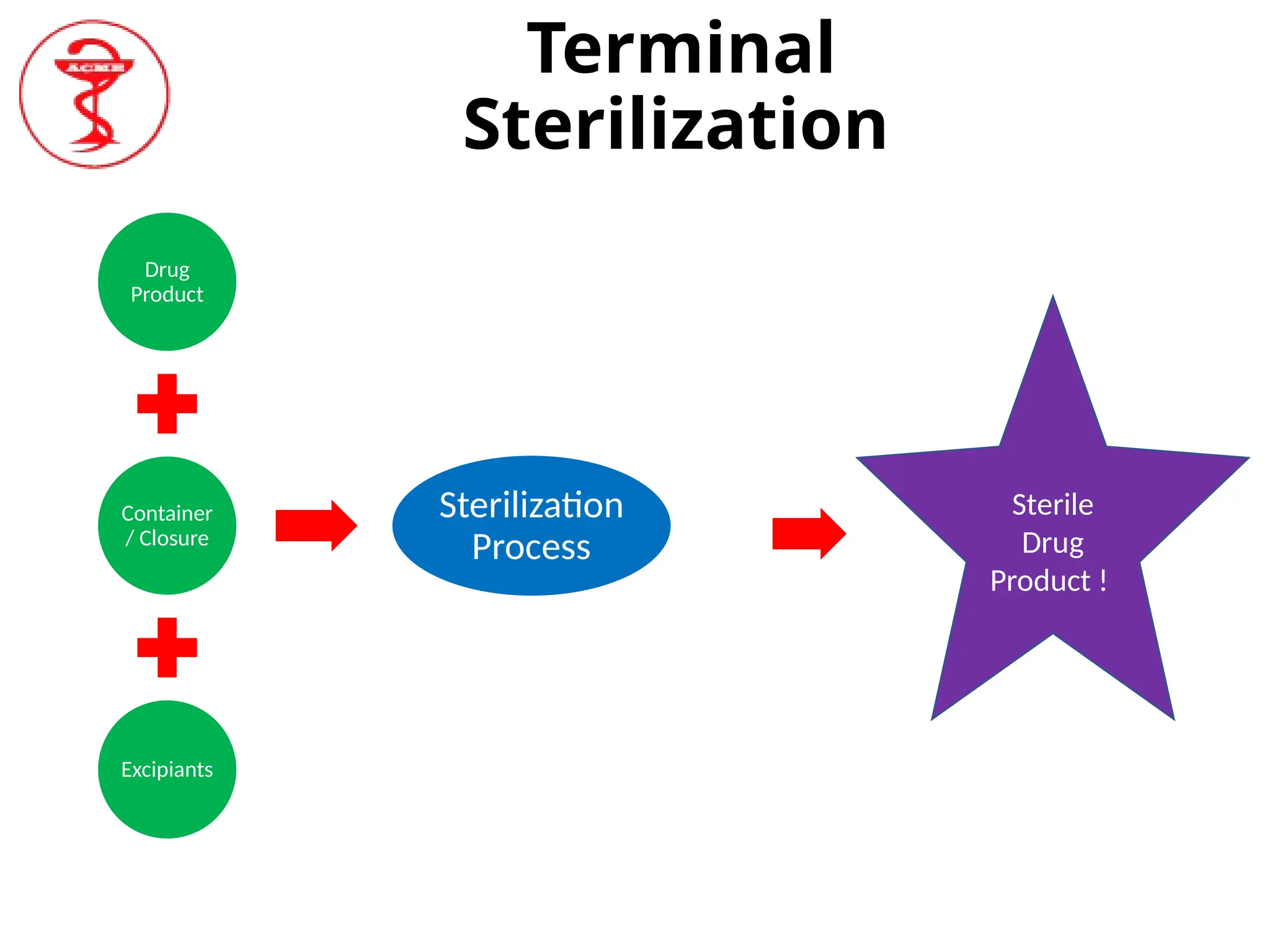
Aseptic processing involves handling sterile products in a controlled environment to minimize contamination, with critical emphasis on filling and sealing containers to maintain sterility. Terminal sterilization processes, such as heat or irradiation, are applied to product containers, which must be done under high-quality environmental conditions due to the lack of sterilization for the final product in its container. The document highlights the significance of controlling microbial contamination, including bacterial spores and endotoxins, and outlines quality control measures such as the LAL assay for endotoxin testing.