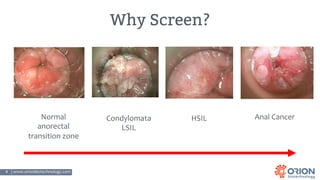
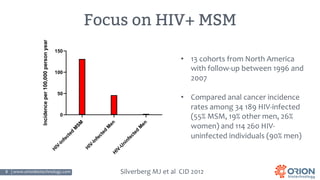
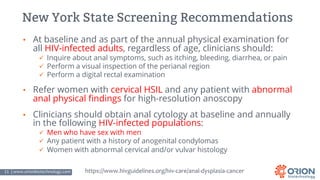
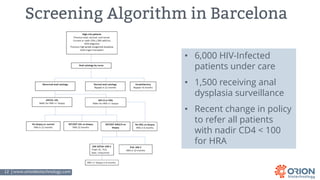
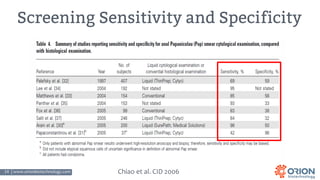
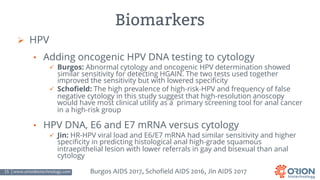
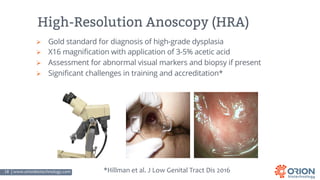
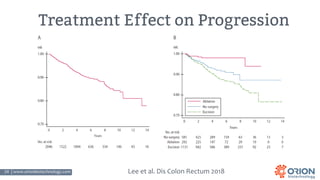
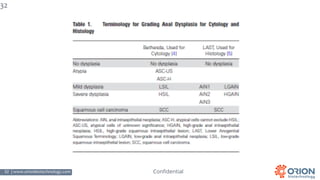
The document discusses the importance of screening and managing anal intraepithelial neoplasia (AIN), particularly among high-risk populations such as HIV-infected men who have sex with men (MSM). It highlights the increased risk of anal cancer in these groups and reviews various screening methods, treatment options, and future study directions aimed at cancer prevention. The evidence supports prioritizing screening and minimally invasive treatments for AIN to reduce anal cancer rates.