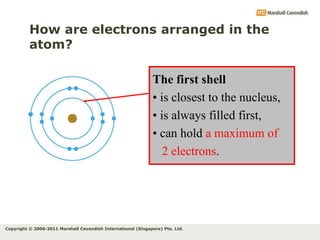
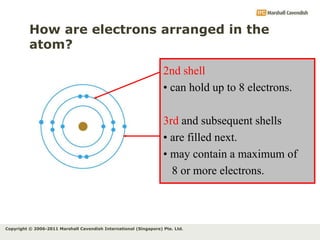
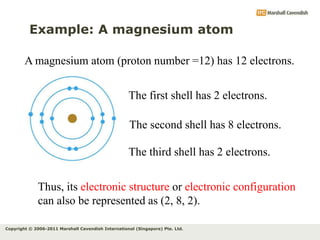
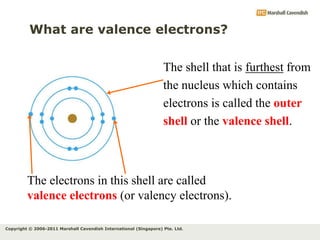
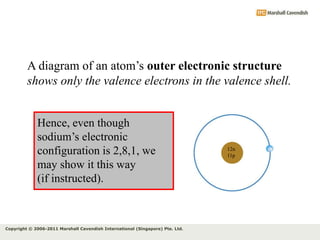
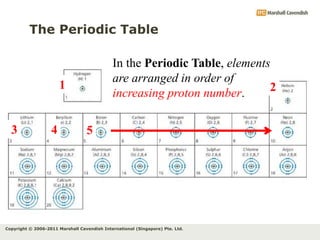
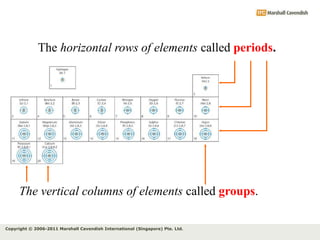
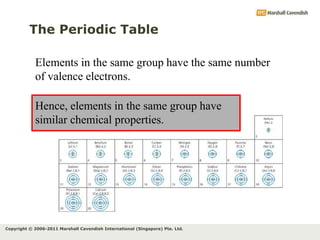
The document discusses the arrangement of electrons in atoms. It states that electrons fill the atomic shells from closest to farthest from the nucleus, with the first shell holding up to 2 electrons and subsequent shells holding up to 8 or more electrons. It defines valence electrons as the electrons in the outermost shell and explains that elements with the same number of valence electrons have similar chemical properties.