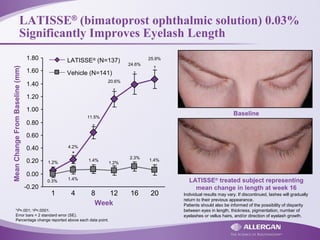
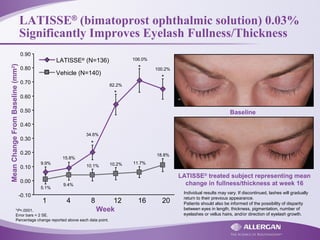
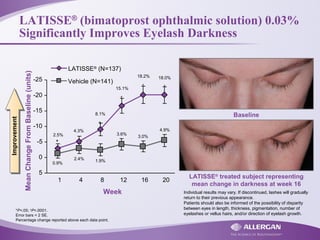
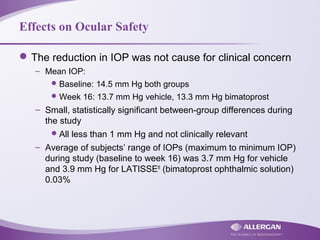
Latisse® (bimatoprost ophthalmic solution) 0.03% is indicated for the treatment of hypotrichosis of eyelashes, enhancing their growth in terms of length, thickness, and darkness. Safety precautions must be followed, including contraindications for hypersensitivity, monitoring for increased intraocular pressure, and potential side effects like iris pigmentation. Proper application using sterile applicators is essential to avoid contamination and unwanted hair growth in unintended areas.





![LATISSE®
(bimatoprost ophthalmic solution) 0.03%
Is a Structural Prostaglandin Analog1
Precise mechanism of action is
unknown
LATISSE®
solution likely
penetrates the hair follicle via
the dermis
– The physicochemical
properties of LATISSE®
solution favor its effective skin
absorption to the dermis
where hair follicles reside
LATISSE®
is believed to exert
its effects by stimulating the
prostamide receptor2,3
1. LATISSE®
[package insert]. Irvine, CA: Allergan, Inc.; 2008; 2. Woodward DF et al. Pharm Ther. 2008;120:71-80;
3. Woodward DF et al. Br J Pharmacol. 2008;153:410-419.
Molecular Structure of LATISSE®
(bimatoprost ophthalmic solution) 0.03%](https://image.slidesharecdn.com/amfastlatissefinal3-3-10-130423135557-phpapp02/85/The-Science-of-Latisse-7-320.jpg)
![LATISSE®
(bimatoprost ophthalmic solution) 0.03%
Increases Overall Eyelash Prominence
Precise mechanism of action is unknown1
1. LATISSE®
[package insert]. Irvine, CA: Allergan, Inc.; 2008; 2. Johnstone MA, Albert DM. Surv Ophthalmol. 2002;47(suppl 1):S185-S202;
3. Elder MJ. Ophthal Plast Reconstr Surg. 1997;13:21-25; 4. Na JI et al. Br J Derm. 2006;155:1170-1176; 5. Data on file. Allergan, Inc.
Stimulates transition
from telogen to
anagen5
Prolongs
anagen1
Normal Eyelash Cycle2-4
Effect of LATISSE®
Percent of
eyelashes in
anagen1
Anagen
≈ 1-2 months
Catagen
≈ 15 days
Telogen
≈ 4-9 months
Exogen](https://image.slidesharecdn.com/amfastlatissefinal3-3-10-130423135557-phpapp02/85/The-Science-of-Latisse-8-320.jpg)



![LATISSE®
(bimatoprost ophthalmic solution) 0.03%
Applicators
Proper use of LATISSE®
requires the accompanying FDA-approved sterile applicators1
The FDA-approved sterile applicators are designed to help patients properly apply the product
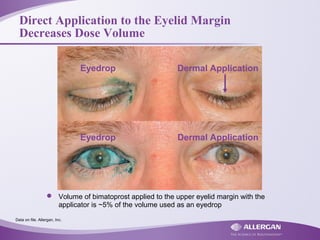
– Volume of bimatoprost when applied to the eyelid margin with the applicator is ≈ 5% of the volume used as
an eyedrop2
Do not apply LATISSE®
in the eye or to the lower lid1
DO NOT APPLY in your eye or to the lower lid because excess hair growth outside the treatment area
may occur. ONLY use the sterile applicators supplied with LATISSE®
to apply the product1
Do not allow the tip of the bottle or applicator to contact surrounding structures, fingers, or any other
unintended surface in order to avoid contamination by common bacteria known to cause infections.1
There
have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical
ophthalmic products
LATISSE®
contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses
should be removed prior to application of LATISSE®
and may be reinserted 15 minutes following its
administration1
It is possible for hair growth to occur in other areas of your skin that LATISSE®
frequently touches. Any
excess solution outside the upper eyelid margin should be blotted with a tissue or other absorbent
material to reduce the chance of this from happening. It is also possible for a difference in eyelash length,
thickness, fullness, pigmentation, number of eyelash hairs, and/or direction of eyelash1
1. . LATISSE®
[package insert]. Irvine, CA: Allergan, Inc 2. Data on file. Allergan, Inc.
Please see Important Safety Information on slides 2-6.](https://image.slidesharecdn.com/amfastlatissefinal3-3-10-130423135557-phpapp02/85/The-Science-of-Latisse-14-320.jpg)
![Iris Pigmentation
Increased iris pigmentation has occurred when the same
formulation of bimatoprost ophthalmic solution
(LUMIGAN®
) was instilled directly into the eye. Although
iridal pigmentation was not reported in clinical studies
with LATISSE®
(bimatoprost ophthalmic solution) 0.03%,
patients should be advised about the potential for
increased brown iris pigmentation which is likely to be
permanent1
No patients (0%) experienced iris pigmentation changes
(dermal application)
Lack of iris pigmentation change in the LATISSE®
trials
likely due to upper eyelid margin application2
1. LATISSE®
[package insert]. Irvine, CA: Allergan, Inc.; 2008; 2. Data on file. Allergan, Inc.](https://image.slidesharecdn.com/amfastlatissefinal3-3-10-130423135557-phpapp02/85/The-Science-of-Latisse-23-320.jpg)







![LATISSE®
(bimatoprost ophthalmic solution) 0.03%:
Increasing the Growth of Eyelashes
LATISSE®
is the first and
only treatment approved
by the FDA indicated to
treat hypotrichosis* of the
eyelashes by increasing
their growth including
length, thickness,
and darkness
*Hypotrichosis is another name for having inadequate or not enough eyelashes.
FDA=US Food and Drug Administration.
LATISSE®
[package insert]. Irvine, CA: Allergan, Inc.](https://image.slidesharecdn.com/amfastlatissefinal3-3-10-130423135557-phpapp02/85/The-Science-of-Latisse-32-320.jpg)