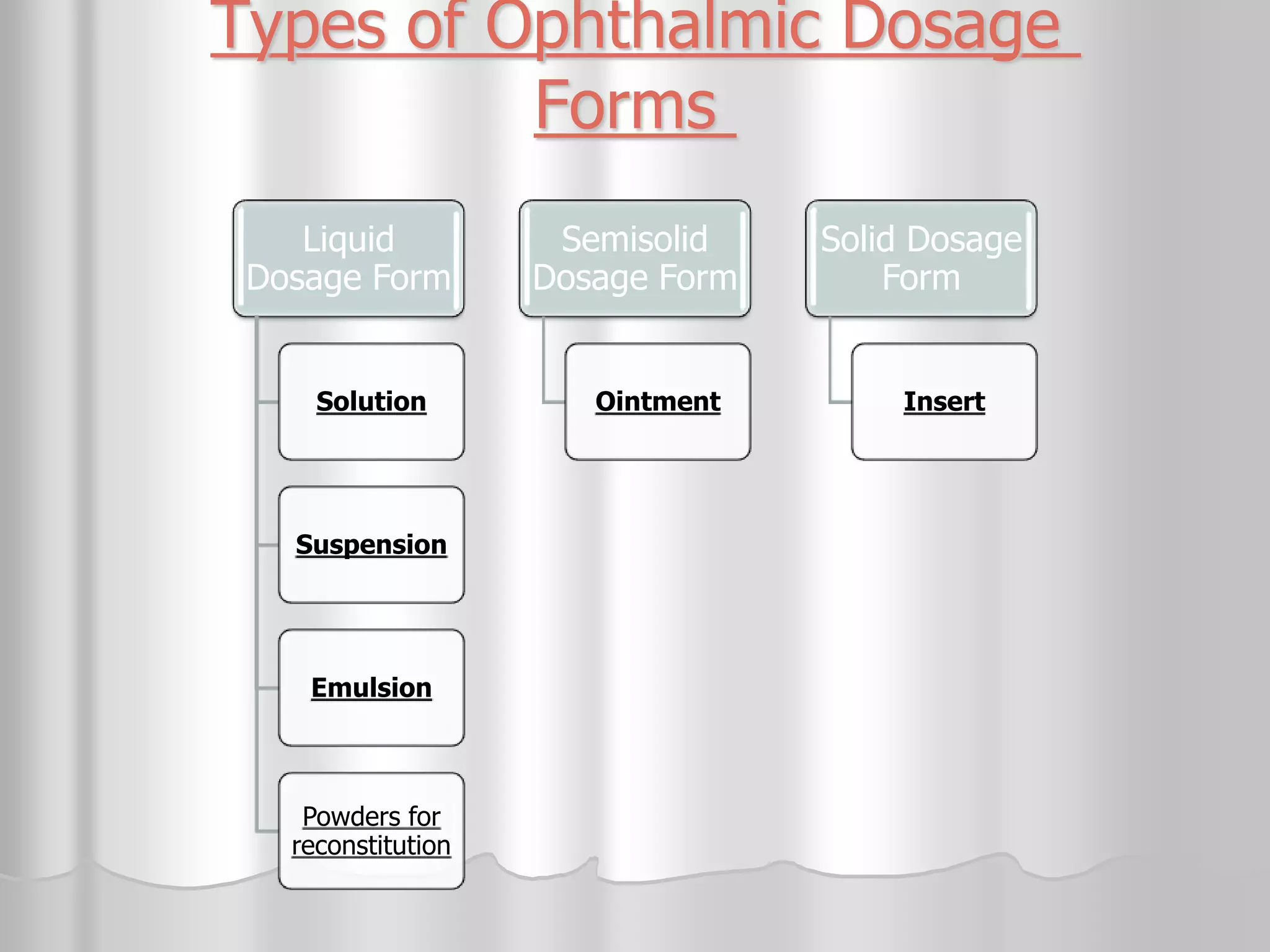
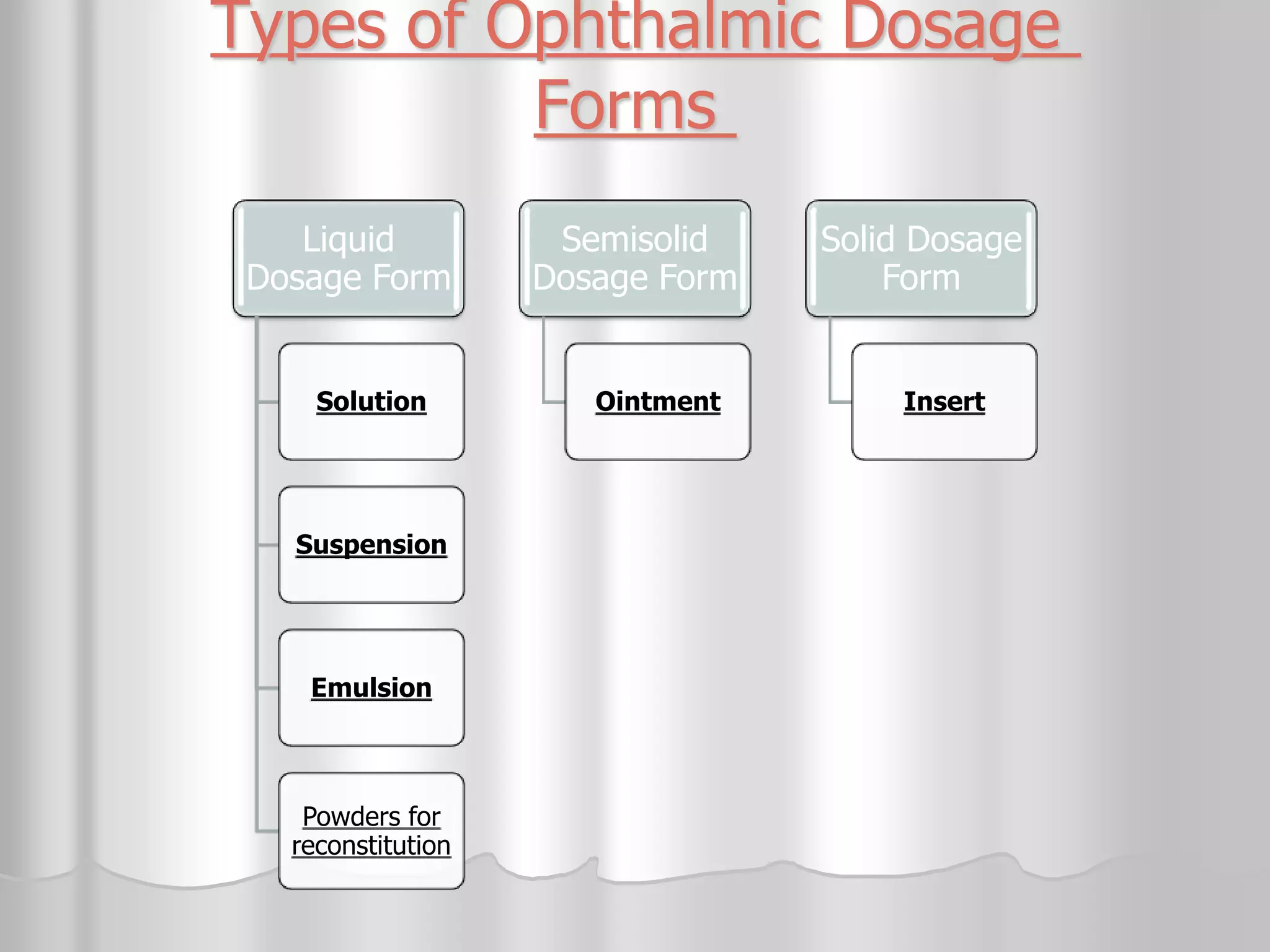
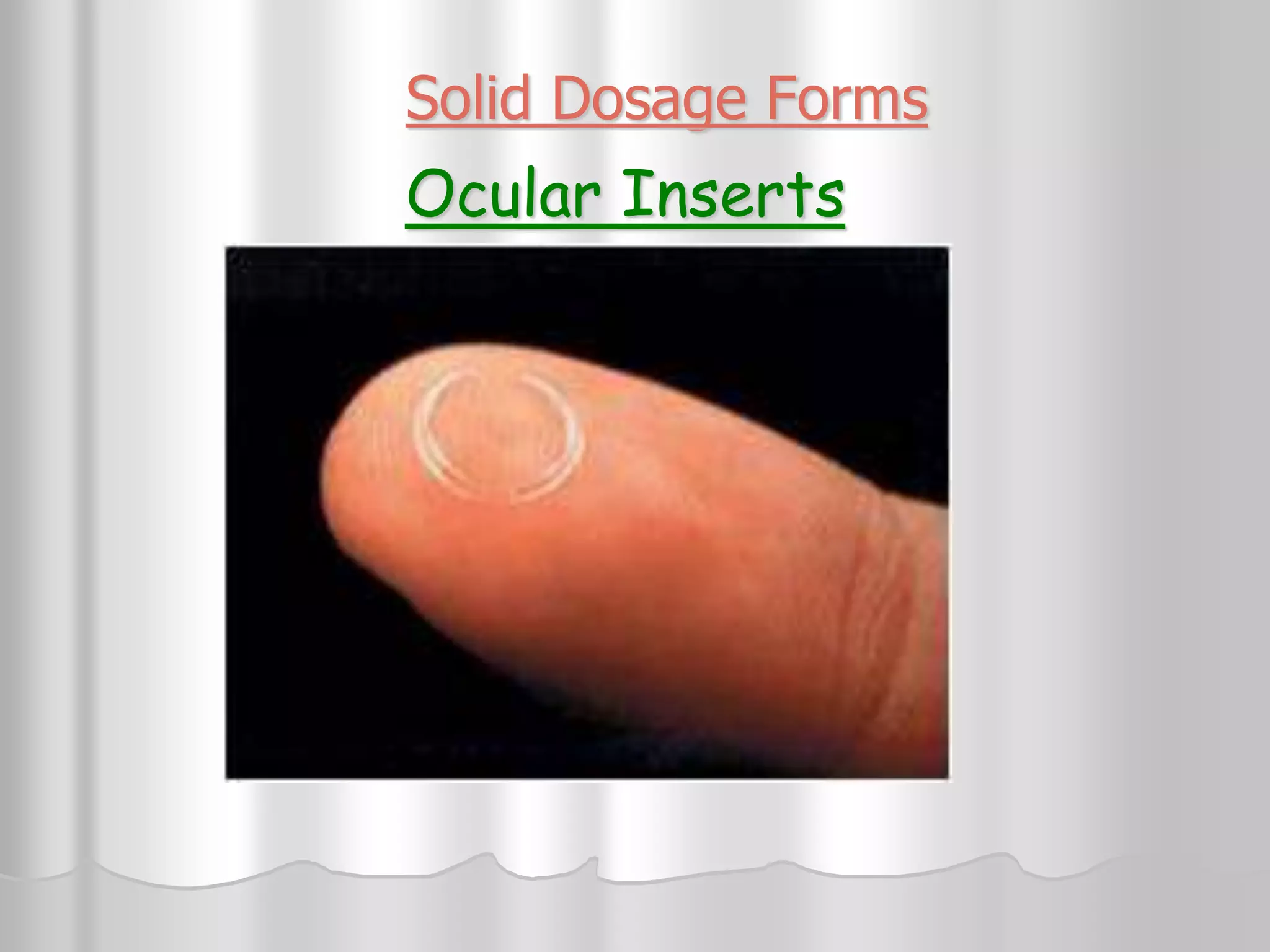
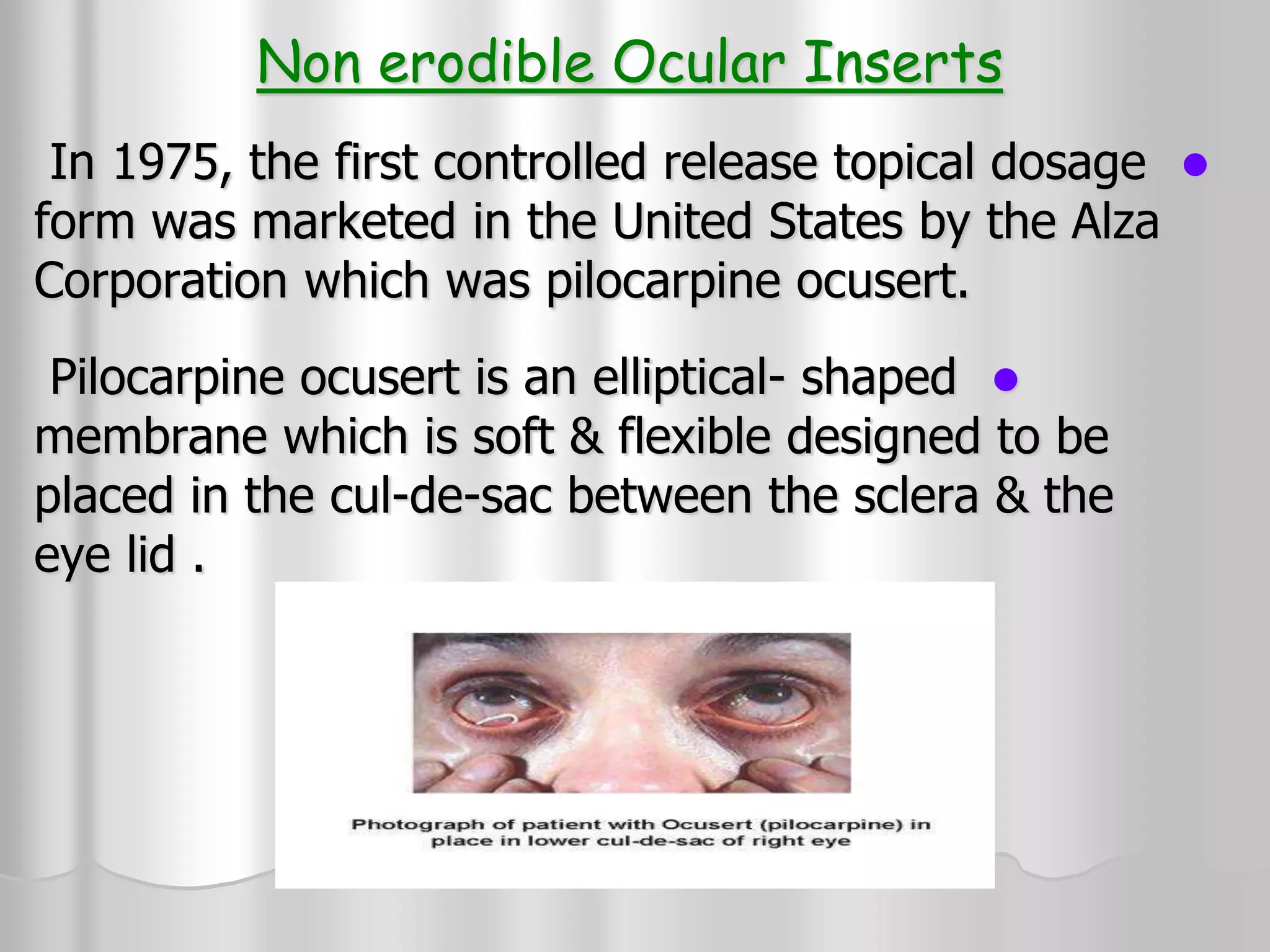
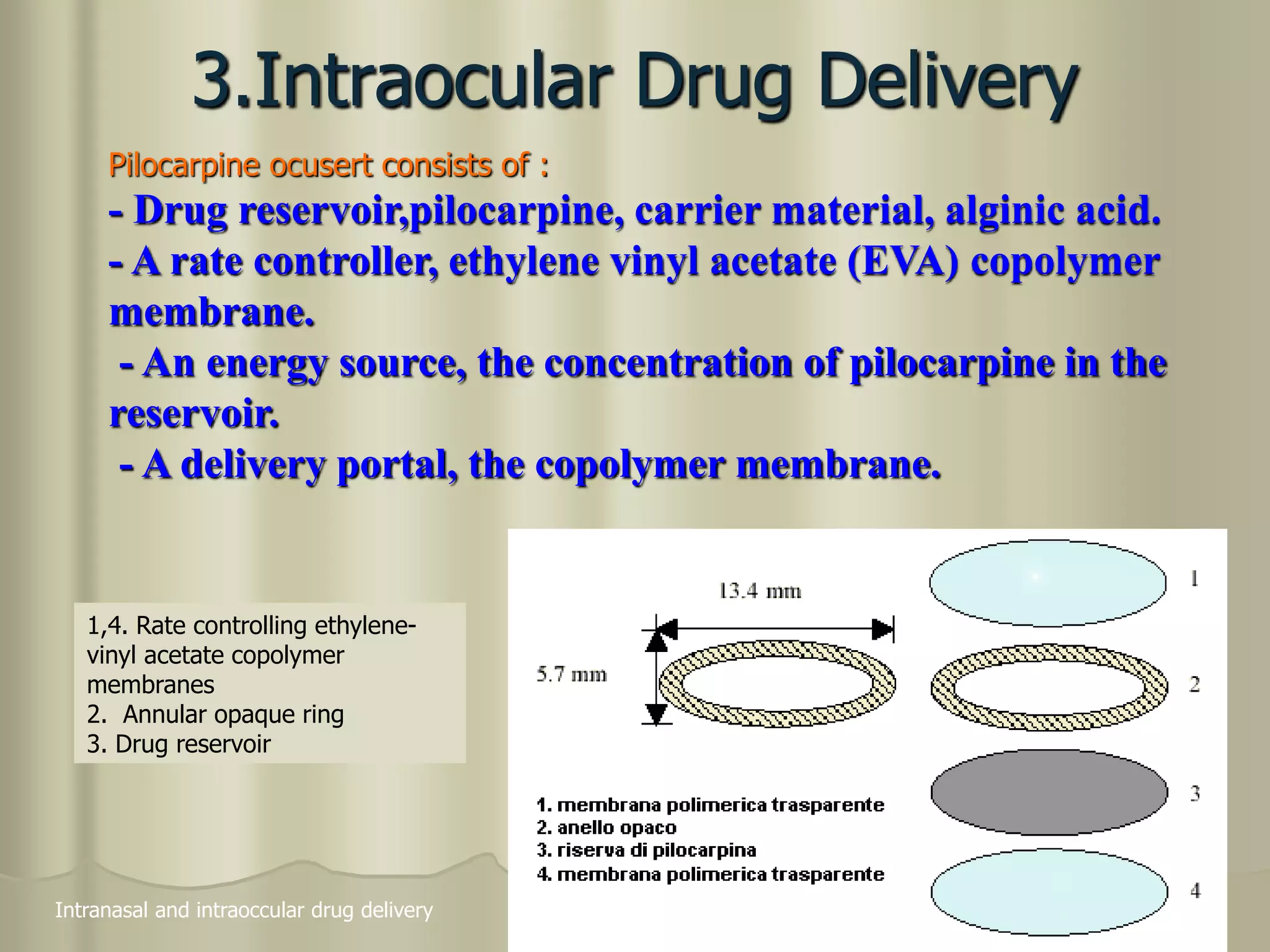
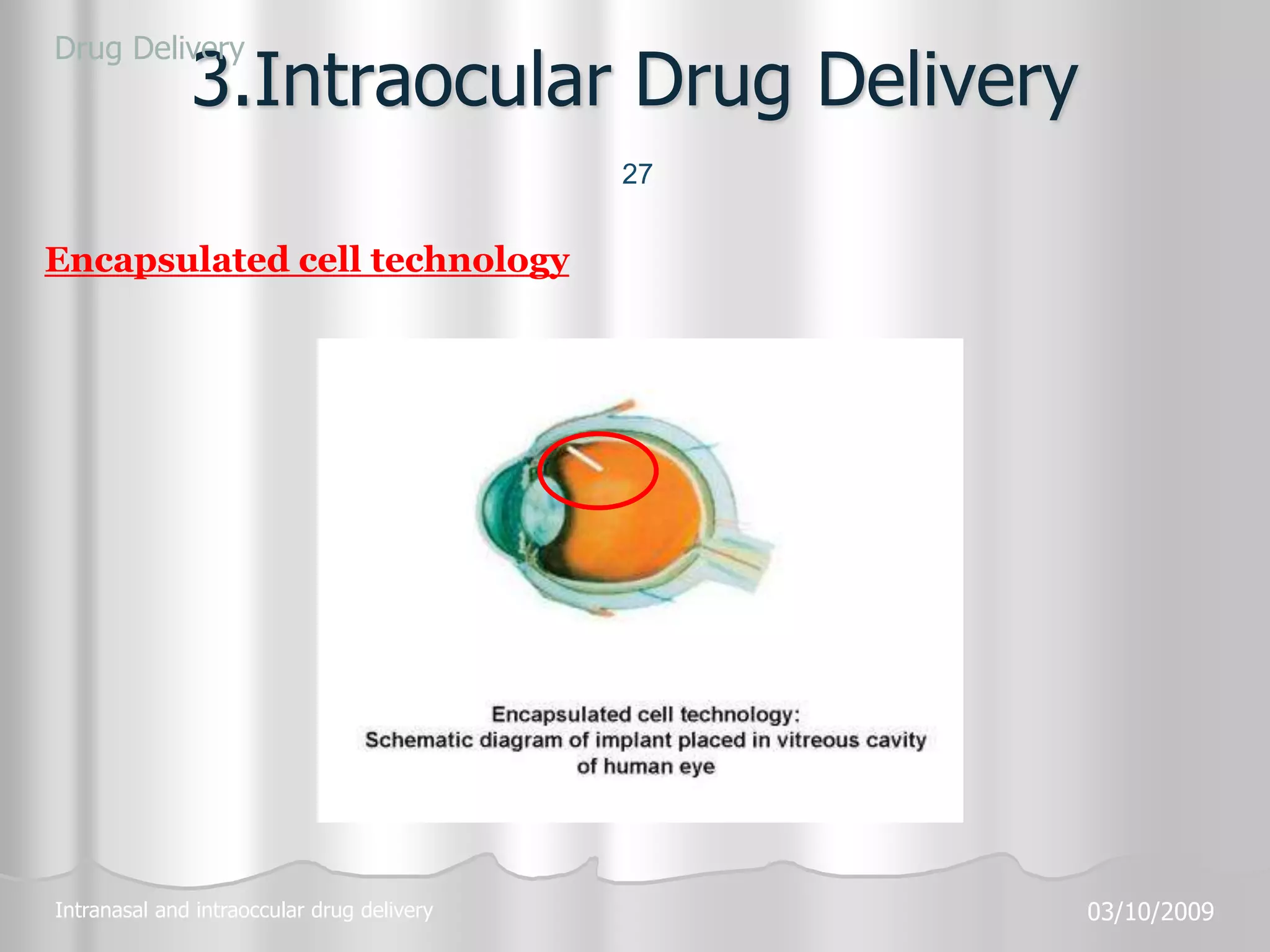
This document discusses various ophthalmic dosage forms including liquid, semisolid, and solid forms. It focuses on describing ophthalmic ointments, inserts, and other semisolid and solid forms. Ophthalmic ointments are typically petrolatum-based and used for antibiotics, antivirals, and corticosteroids. Inserts like Pilocarpine Ocusert provide controlled release of drugs over time. Intracmeral injections and implants are also discussed as methods for intraocular drug delivery.