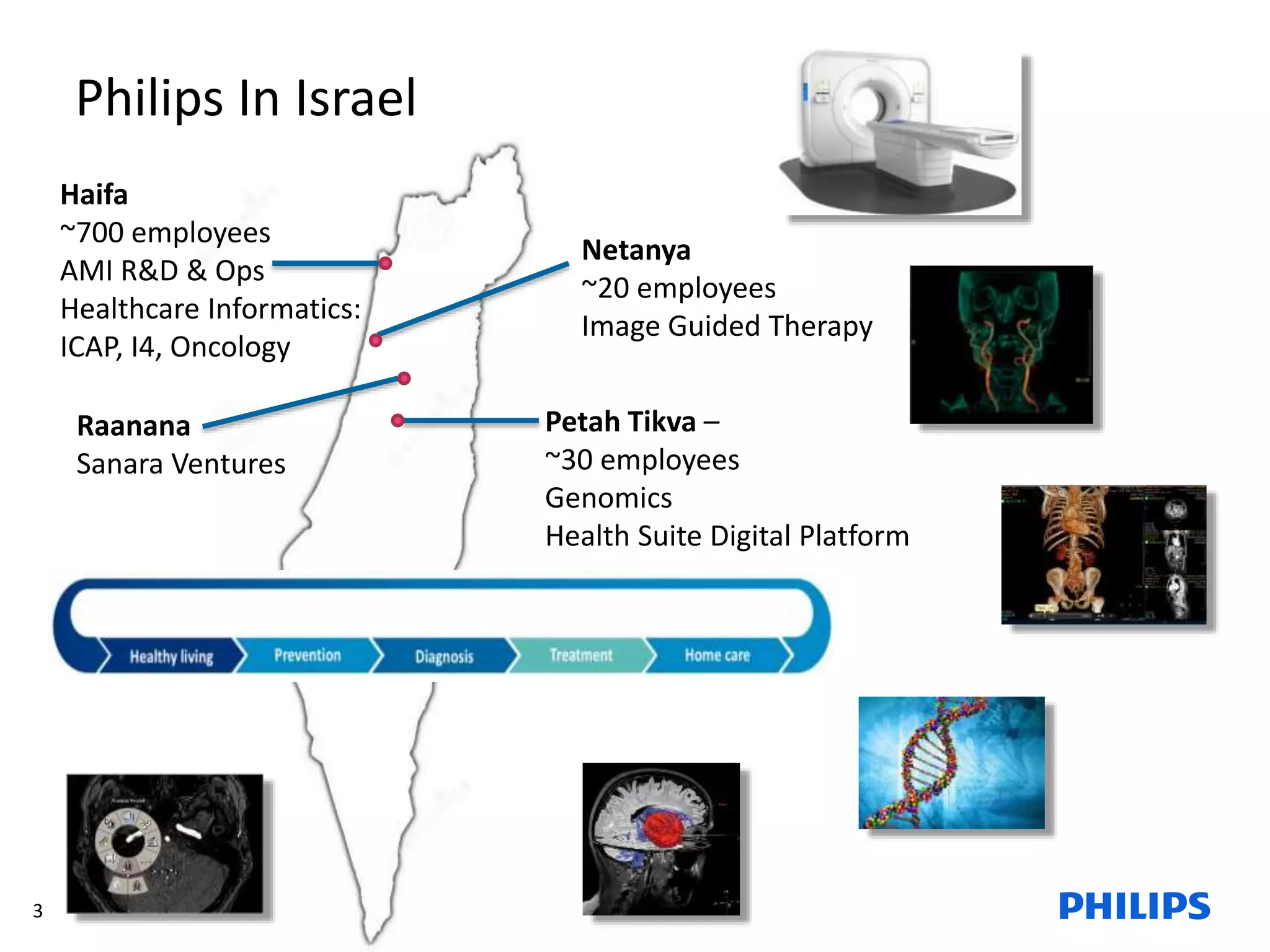
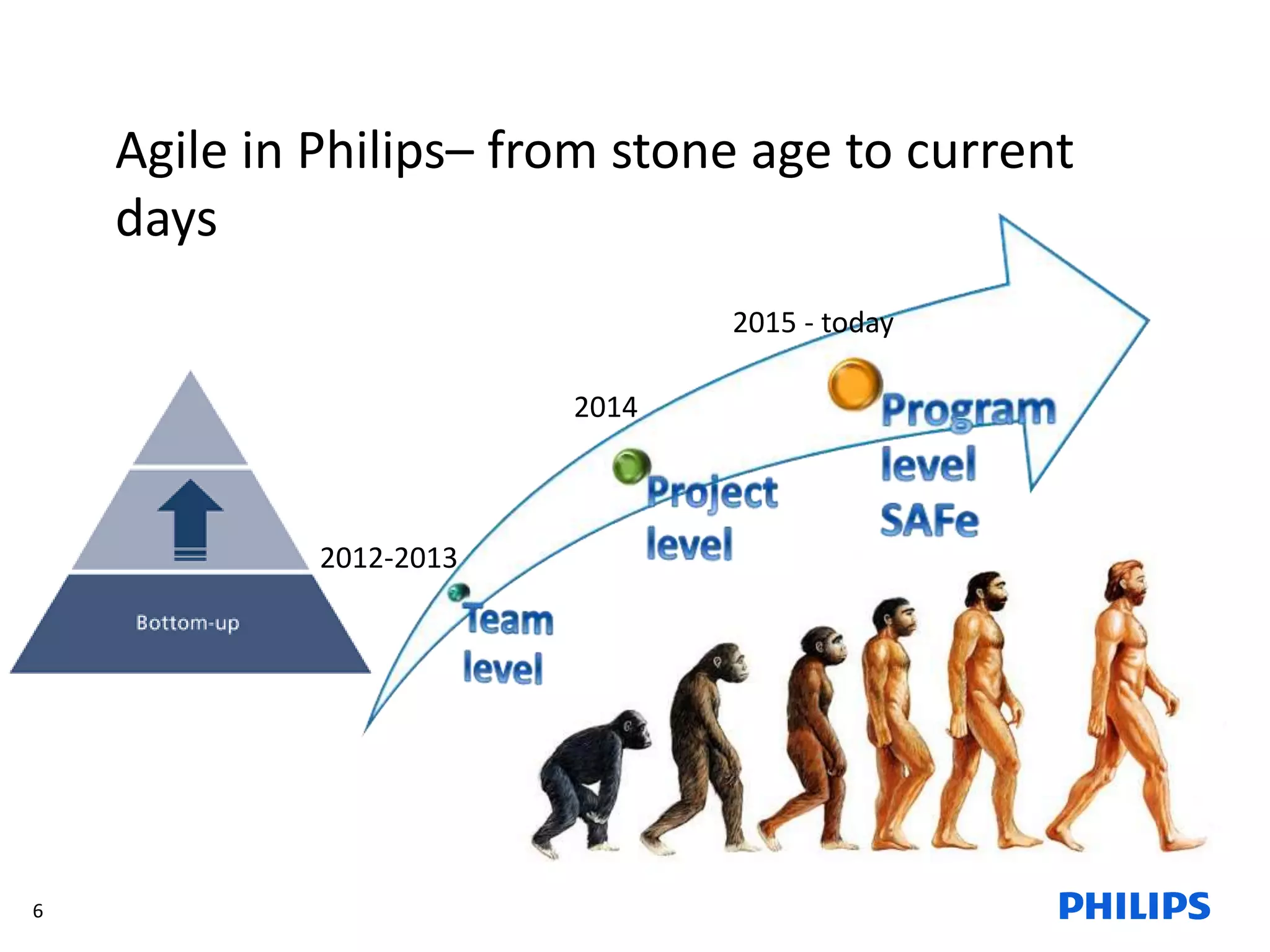
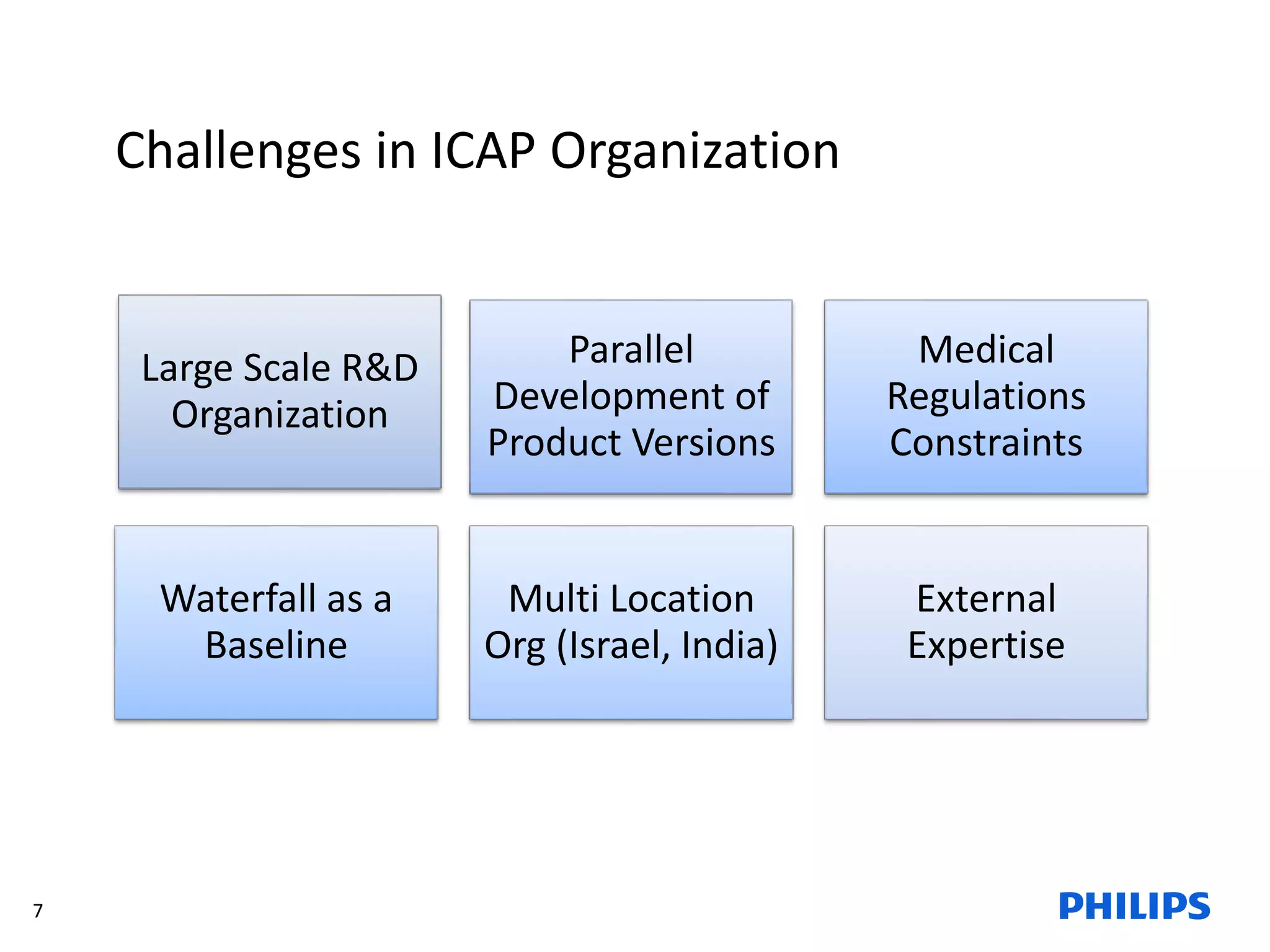
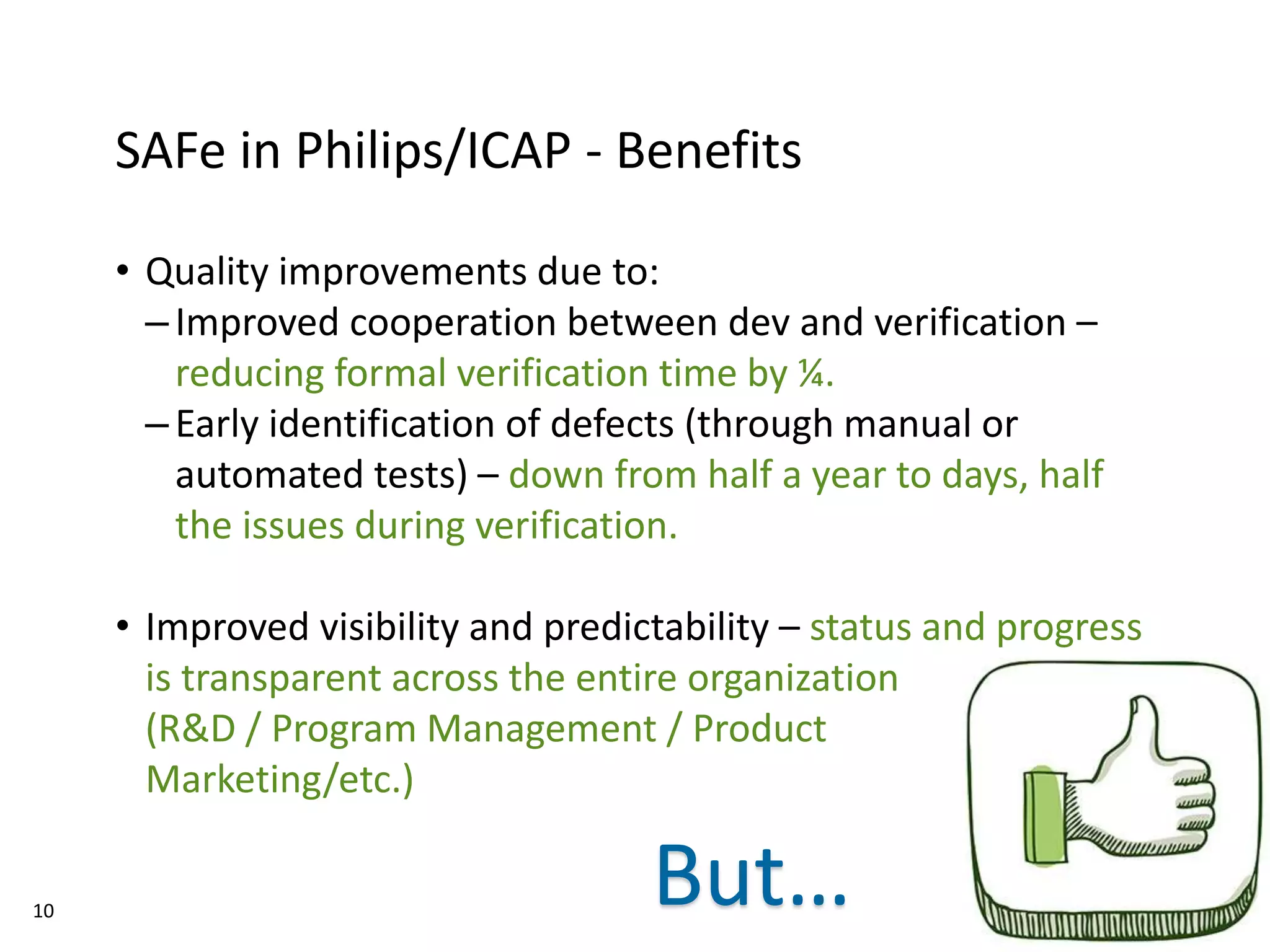
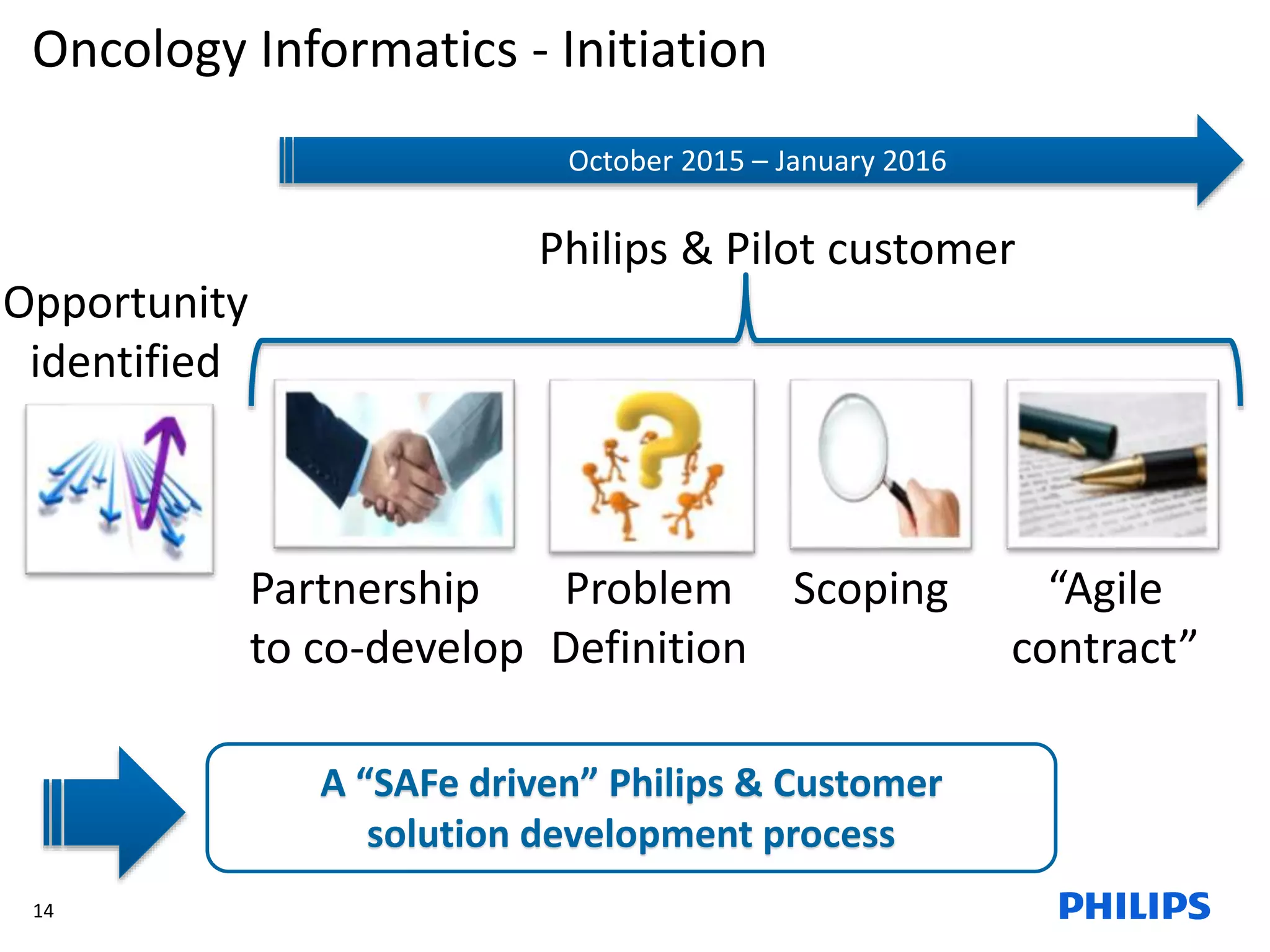
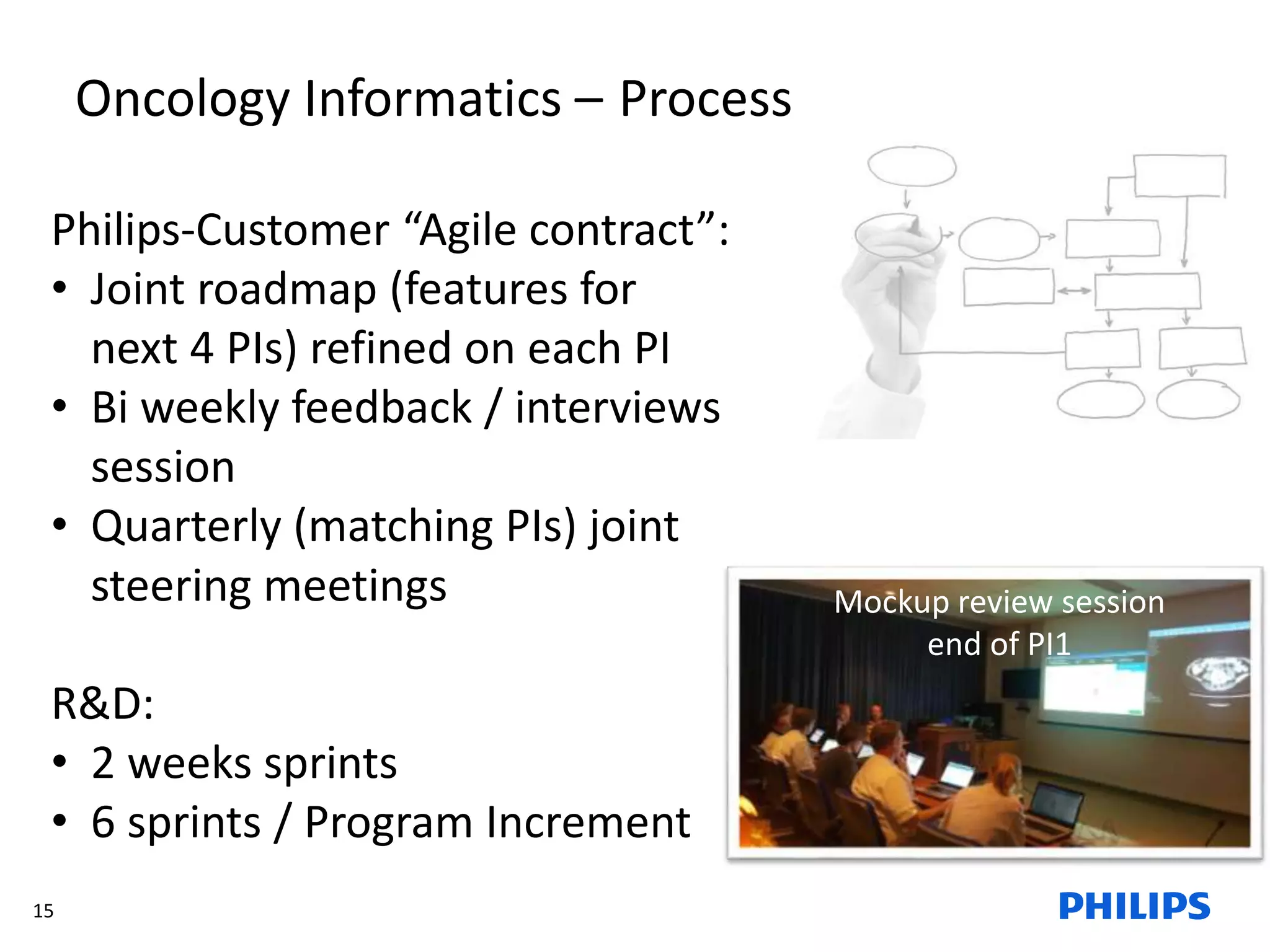
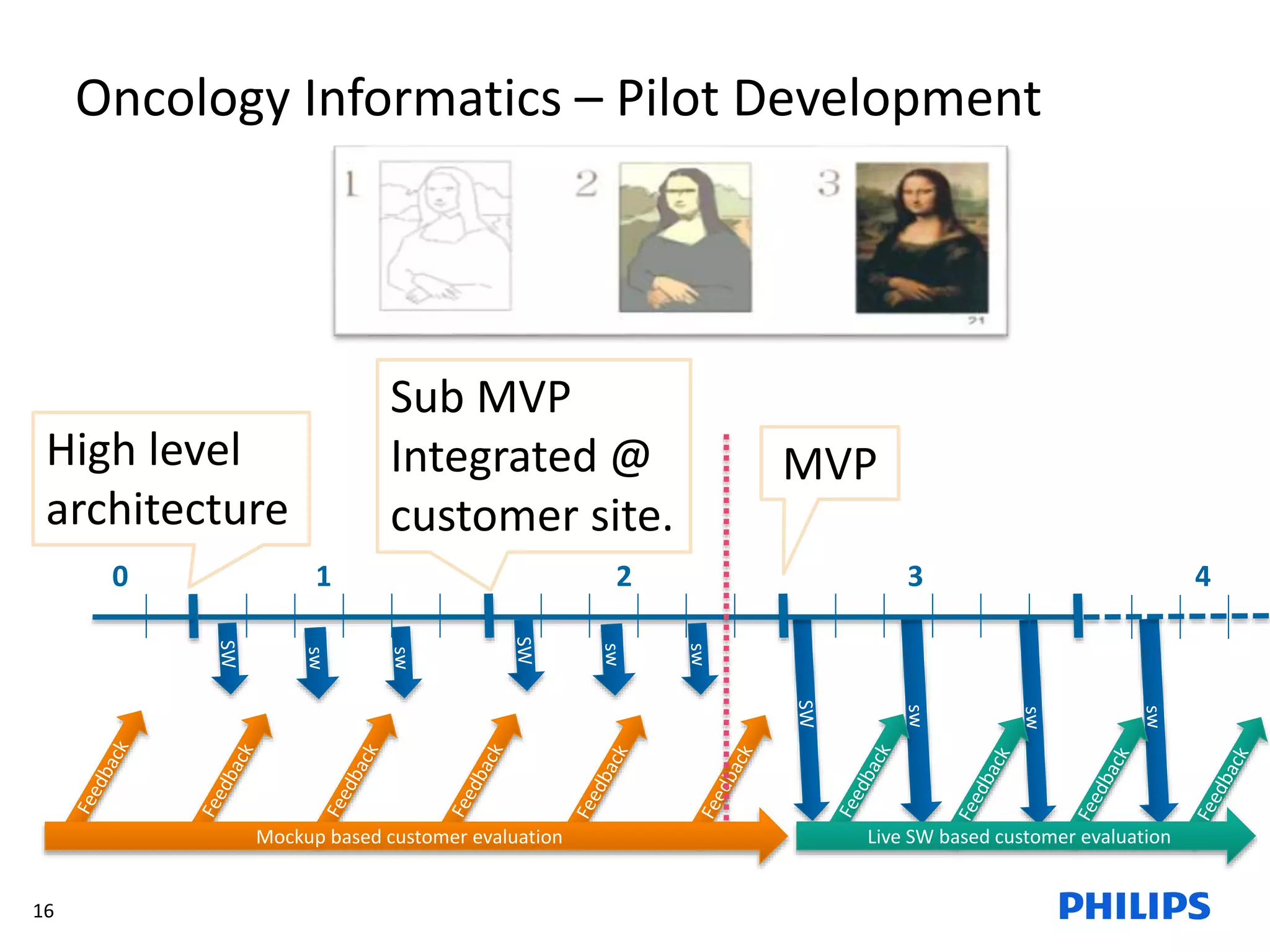
The document discusses Philips ICAP's transition to an Agile framework, highlighting the implementation of the SAFe methodology to improve product quality and feedback loops in a large-scale R&D organization. It outlines the benefits realized, such as faster feedback from customers and improved visibility across teams, while also addressing challenges like regulatory constraints and logistical issues during planning. The document also mentions ongoing focus areas for further alignment and standardization of Agile practices within the organization.