This document provides information about open esophageal surgical procedures, including cricopharyngeal myotomy and excision of Zenker's diverticulum. It describes the preoperative evaluation and optimization of patients, including imaging, endoscopy, and nutritional support. The surgical technique is explained in 4 steps: 1) incision and dissection of the pharyngeal pouch, 2) myotomy of the cricopharyngeus muscle and esophagus, 3) freeing or excising the diverticulum using a stapler, and 4) drainage/closure. Postoperative care involves monitoring for complications such as recurrent laryngeal nerve injury, fistula, hematoma, and infection.
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 1
7 OPEN ESOPHAGEAL PROCEDURES
John Yee, M.D., F.R.C.S.C., and Richard J. Finley, M.D., F.A.C.S., F.R.C.S.C.
The remarkable developments in diagnosis, imaging, and surgical nuclear studies for assessment of esophageal and gastric transit
treatment of esophageal diseases over the past 15 years have result- provide functional data that can facilitate the diagnosis and treat-
ed in markedly better patient outcomes: the morbidity and mor- ment of GERD, achalasia, and other disorders of the esophagus.
tality associated with surgery of the esophagus have been substan- They are useful complements to standard investigations (e.g., ciné
tially reduced. In particular, the operative techniques employed to barium swallow and endoscopy).
treat esophageal disease have advanced considerably, as a result of Complete preoperative investigation of all patients, even those
an improved understanding of esophageal anatomy and physiolo- with classic histories and physical findings, is mandatory.The data
gy and the successful introduction of minimally invasive approach- from anatomic and functional testing allow the surgeon to plan the
es to the esophagus [see 4:8 Minimally Invasive Esophageal Procedures]. operation more appropriately and effectively (e.g., deciding on the
For a number of diseases (e.g., achalasia), minimally invasive pro- need for esophageal lengthening in patients with paraesophageal
cedures have proved to be as effective as their open counterparts hernias or choosing between a complete and a partial fundoplica-
while causing less postoperative morbidity.The growing stature of tion in patients with hernias associated with varying degrees of
minimally invasive approaches does not, however, diminish the im- esophageal dysmotility).
portance of the equivalent open approaches. In this chapter, we
OPTIMIZATION OF PATIENT HEALTH STATUS
describe common open operations performed to excise Zenker’s
diverticulum, to manage complex gastroesophageal reflux disease Patients with obstructing esophageal diseases are often elderly,
(GERD), and to resect esophageal and proximal gastric tumors. debilitated, and malnourished. Although months of insufficient
nutrition cannot be corrected in the space of a few hours, anemia,
dehydration, and electrolyte abnormalities can be mitigated by
General Preoperative Considerations means of intravenous support and appropriate laboratory monitor-
ing. If esophageal obstruction prevents oral intake, endoscopic dila-
METHODS OF PATIENT ASSESSMENT
tion of the stricture, accompanied by either nasogastric intubation
The functional results achieved with esophageal procedures or percutaneous endoscopic gastrostomy (PEG) [see 5:18 Gastro-
become more predictable when the approach to preoperative intestinal Endoscopy], is indicated; the patient should then be able to
patient evaluation is precise and reproducible. The ciné barium resume at least a liquid diet. If weight loss has exceeded 10%, enter-
swallow remains the most cost-effective method for initial evalua- al nutrition, comprising at least 2,000 kcal/day of a high-protein liq-
tion of esophageal anatomy and function. It should be employed uid diet, should be administered for at least 10 days before the oper-
before endoscopy because the results may direct the endoscopist’s ation. Cardiovascular, renal, hepatic, and respiratory function should
attention to particular areas of concern. For example, a finding of be documented and optimized. If the patient is aspirating, the esoph-
abnormal angulation or strictures indicates that the endoscopist agus should be evacuated and the patient should be given nothing
should either use a pediatric-caliber endoscope or exercise more by mouth until after the operation. Aspiration pneumonia should
caution in passing a standard adult endoscope. In addition, endo- always be corrected preoperatively.
scopic examination alone is often insufficient for assessing
esophageal motility disorders or defining the complex anatomy of
a paraesophageal hiatal hernia. Cricopharyngeal Myotomy and Excision of Zenker’s
Endoscopic ultrasonography (EUS) is an extension of the Diverticulum
visual mucosal examination. The information it can provide
PREOPERATIVE EVALUATION
about the extension of mass lesions beyond the confines of the
esophageal wall is helpful in planning surgical resection. In addi- Patients who are candidates for cricopharyngeal myotomy usu-
tion, EUS can differentiate benign stromal tumors from cystic or ally present with difficulty initiating swallowing, cervical dysphagia
malignant neoplasms on the basis of characteristic echogenicity or odynophagia [see 4:1 Dysphagia], and a history of pulmonary
patterns. The combination of EUS and computed tomography aspiration. These symptoms of cricopharyngeal dysfunction may
permits highly precise anatomic assessment of esophageal neo- or may not be associated with a Zenker’s diverticulum. Ciné con-
plasms, definition of the extent of local invasion, and identifica- trast studies may reveal poor pharyngeal contractility, pulmonary
tion of regional metastases. or nasal aspiration, abnormalities of the upper esophageal sphinc-
Functional imaging with photodynamic or vital staining allows ter, pharyngeal pouches, or other structural abnormalities in the
accurate diagnosis of dysplastic or malignant mucosal lesions in distal esophagus. Barium is the usual contrast agent, but if aspira-
their earliest stages. Positron emission tomography (PET) yields tion is suspected, a nonionic contrast agent can be used instead to
similar results by localizing metabolically active tissue regionally or prevent pneumonitis.
at distant sites. The combination of morphologic data from high- Zenker’s diverticulum is a pulsion diverticulum that arises
resolution CT and functional data from PET is particularly effec- adjacent to the inferior pharyngeal constrictor, between the
tive for identifying occult metastases that would preclude curative oblique fibers of the posterior pharyngeal constrictors and the
resection for esophageal cancer. cricopharyngeus muscle. This mucosal outpouching results from
Esophageal manometry, 24-hour esophageal pH testing, and a transient incomplete opening of the upper esophageal sphinc-](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-1-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 2
roll is placed behind the shoulders to extend the neck.The patient
is placed in a 20º reverse Trendelenburg position, and the legs are
wrapped with pneumatic calf compressors to prevent deep vein
thrombosis (DVT).With the endotracheal tube placed to the left
side of the mouth, a preliminary flexible esophagogastroscopy is
performed to empty the diverticulum of all food and to examine
the esophagus and the stomach. The scope is then brought back
up into the oropharynx and moved into the pouch. The location
of the diverticulum (on the left or the right side) is confirmed by
turning off the room lights and noting which side is transillumi-
nated by the gastroscope.
OPERATIVE TECHNIQUE
Step 1: Incision and Dissection of Pharyngeal Pouch
The patient lies with the head turned away from the side on
which the incision is made. The cricoid cartilage is palpated and
marked. A 4 cm skin incision is made, either obliquely along the
sternocleidomastoid muscle [see Figure 1] or transversely in a skin
crease at the level of the cricoid. The platysma is divided in the
same line. Self-retaining retractors are inserted. The anterior bor-
der of the sternocleidomastoid muscle is incised throughout its
length.The omohyoid muscle and the sternohyoid and sternothy-
roid muscles are retracted [see Figure 2]. The sternocleidomastoid
muscle is retracted laterally to expose the carotid sheath and the
Figure 1 Cricopharyngeal myotomy and excision of Zenker’s internal jugular vein.The middle thyroid vein is ligated and divid-
diverticulum. A soft roll is placed behind the shoulders to extend ed, and the thyroid gland and the trachea are retracted medially by
the neck. The head is turned to the side opposite the incision. The
the assistant’s finger to minimize the risk of injury to the underly-
cricoid cartilage is palpated and marked. The skin is incised
obliquely along the sternocleidomastoid muscle, as shown, or
ing recurrent laryngeal nerve. There is no need to encircle the
transversely in a skin crease at the level of the cricoid. esophagus or to dissect in the tracheoesophageal groove.The deep
cervical fascia is divided. The inferior thyroid artery is divided as
laterally as possible. The carotid sheath is retracted laterally, and
ter. The diverticulum ultimately enlarges, drapes over the crico-
pharyngeus, and dissects behind the esophagus into the prever-
tebral space.The pouch usually deviates to one side or the other;
Thyroid Gland
accordingly, the side on which the deviation occurs must be
determined by means of a barium swallow so that the appropri- Omohyoid Muscle
ate operative approach can be selected. Esophageal motility stud-
ies may show either incomplete upper esophageal relaxation on
swallowing or poor coordination of the upper esophageal relax-
ation phase with pharyngeal contractions. Upper GI endoscopy
is performed preoperatively to exclude the presence of a pharyn-
geal or esophageal carcinoma and to assess the upper GI anato-
my. If there is evidence of GERD, proton pump inhibitors (PPIs)
are given.
In symptomatic patients (e.g., those with dysphagia, nocturnal
cough, or recurrent pneumonia from aspiration), surgical therapy
is indicated regardless of whether a pouch is present or how large
it may be. Such treatment involves correcting the underlying cri- Inferior
copharyngeal muscle dysfunction with a cricopharyngeal myoto- Thyroid
my. If there is a diverticulum larger than 2 cm, it should be excised Artery
in addition to the cricopharyngeal myotomy. Alternatively, the
diverticulum may be managed via endoscopic obliteration of the
common wall between the pharyngeal pouch and the esophagus
with either a stapler or a laser. Cricopharyngeal incoordination
may be temporarily relieved by injecting botulinum toxin into the
cricopharyngeus.
Figure 2 Cricopharyngeal myotomy and excision of Zenker’s
OPERATIVE PLANNING diverticulum. The sternocleidomastoid is incised along the ante-
rior border so as to expose the omohyoid muscle and the ster-
The patient is placed on a clear fluid diet for 2 days before the nohyoid and sternothyroid muscles, which are retracted. The thy-
operation. With the patient under general anesthesia, the trachea roid gland and the trachea are retracted medially by the assis-
is intubated with a single-lumen endotracheal tube. Cricoid pres- tant’s finger, and the inferior thyroid artery is ligated and divided
sure is applied to prevent aspiration of diverticular contents. A soft laterally to avoid injury to the recurrent laryngeal nerve.](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-2-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 3
a b
Left
Recurrent
Laryngeal
Nerve
Figure 3 Cricopharyngeal myotomy and excision of Zenker’s diverticulum. (a) The diverticulum is dis-
sected away from the esophagus, and an esophageal myotomy is started approximately 3 cm below the
cricopharyngeus. The myotomy is continued proximally through the cricopharyngeus, and the muscle
around the diverticulum is freed. (b) A linear stapler is placed at the base of the sac and pressed firmly
against the esophagoscope. The stapler is fired, and the diverticulum is excised.
dissection is carried down to the prevertebral fascia [see Figure 2]. to injure the recurrent laryngeal nerve.The stapler is fired, and the
The endoscope placed in the diverticulum is palpated, and the diverticulum is excised.The staple line is cleaned with an antisep-
pouch is dissected away from the cervical esophagus up as far as the tic solution, and the incision is filled with saline.The esophagus is
pharyngoesophageal junction. The flexible endoscope is then insufflated with air to determine whether mucosal leakage has
removed from the pouch and advanced into the thoracic esopha- occurred, and the esophagoscope is removed; any mucosal leaks
gus so that it can be used as a stent for the cricopharyngeal myoto- found are closed with fine absorbable sutures. In the absence of a
my. Dissection of the pharyngeal pouch is then completed. stapler, the best way of excising the sac is to make a series of short
incisions through the neck of the sac with scissors, suturing the
Step 2: Myotomy edges after each cut with absorbable monofilament sutures (the
The esophageal myotomy is started approximately 3 cm below so-called cut-and-sew technique). The esophagoscope ensures
the cricopharyngeus on the posterolateral esophageal wall [see that the esophageal lumen is not narrowed.
Figure 3a].The esophageal muscle is divided down to the mucosa,
which is recognizable from its bluish coloration with the submu- Step 4: Drainage and Closure
cosal plexus overlying it.The esophageal muscle is dissected away Once hemostasis has been achieved, a short vacuum drain is
from the mucosa with a right-angle dissector and divided with a placed through the skin into the retroesophageal space.The platys-
low-intensity diathermy unit. The myotomy is then continued ma is repaired with absorbable sutures, and the skin is closed with
proximally through the cricopharyngeus and up into the muscular a subcuticular absorbable suture. Nasogastric intubation is unnec-
wall of the hypopharynx for 2 cm if there is no diverticulum pres- essary. Prokinetic agents and PPIs are administered to prevent
ent. The hypopharynx is distinguished by a pronounced submu- gastroesophageal reflux. A water-soluble contrast study is done on
cosal venous plexus. The muscle is then swept off the mucosa for the day of the operation. If the results are normal, the patient is
120°. started on a liquid diet, and the drain is removed on postoperative
day 1, when the patient is discharged.
Step 3: Freeing or Excision of Diverticulum
COMPLICATIONS
If there is a diverticulum less than 2 cm in diameter, the
cricopharyngeus is transected and the muscularis around the The main complications associated with cricopharyngeal myo-
diverticulum is freed. The myotomy is extended onto the tomy are recurrent laryngeal nerve trauma (occurring in 0.5% of
hypopharynx for 2 cm.The diverticulum may be suspended to the cases), fistulas (1%), hematoma formation, infection (2%), aspiration,
back wall of the pharynx. It should not be sutured to the prever- and recurrence (4%). Hematomas and infections must be drained
tebral fascia, because the passage of sutures through the divertic- promptly. Fistulas usually close once the prevertebral space is drained
ulum can contaminate the fascia, leading to an increased risk of and the associated infection controlled. Aspiration is the most
fascial infection. serious complication after cricopharyngeal myotomy. Gastroesoph-
If the diverticulum is more than 2 cm in diameter, it is excised ageal reflux may contribute to oropharnygeal dysphagia. Division
with a linear stapler loaded with 2.5 mm staples, which is placed of the upper esophageal sphincter in a patient with an incompetent
at the base of the sac and pressed firmly against the esophagoscope esophagogastric junction may lead to massive tracheobronchial
[see Figure 3b]. Particular care must be taken at this point so as not aspiration. Therefore, documented gastroesophageal reflux, gas-](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-3-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 4
troesophageal regurgitation, and severe distal esophagitis may be the most appropriate form of repair is. Specifically, a history of
relative contraindications to cricopharyngeal myotomy until the heartburn and effortless regurgitation should be sought. Dysphagia
lower esophageal sphincter defect has been remedied with an anti- and odynophagia are not typically associated with hiatal hernia
reflux operation. unless there is a significant paraesophageal component. Persistent
dysphagia may reflect the presence of a stricture or a neoplasm [see
OUTCOME EVALUATION
4:1 Dysphagia]. Reflux-induced esophageal spasm may present
Of patients with a Zenker’s diverticulum, at least 90% experi- with occasional episodes of cervical dysphagia, but the transient
ence excellent results from surgical treatment. Of patients with- nature of the symptoms easily differentiates this condition from
out a Zenker’s diverticulum, one third experience excellent dysphagia caused by a fixed obstruction. Chest pain that radiates
results, another third show moderate improvement, and the remain- toward the back after meals and is relieved by nonbilious vomiting
ing third show no improvement.1 Patients with poor pharyngeal may indicate the presence of an incarcerated intrathoracic stomach
contractility in conjunction with normal upper esophageal that is hindering the emptying of the paraesophageal component.
sphincter function show little improvement with cricopharyngeal Atypical chest pain from cholelithiasis, peptic ulcer, or coronary
myotomy. Patients with oropharyngeal dysphagia secondary to artery disease may confound the diagnosis.
neurologic involvement who have intact voluntary deglutination,
adequate pulsion of the tongue, and normal phonation may Imaging
show improvement with cricopharyngeal myotomy. Appropriate Radiographic investigation should begin with a ciné barium
selection of patients for cricopharyngeal myotomy leads to bet- swallow, which will yield valuable information regarding the length
ter surgical outcomes. of the esophagus, its peristaltic function, and the integrity of the
mucosal surface. The gastric views can be used for qualitative
assessment of distal emptying. Any paraesophageal component
Transthoracic Hiatal Hernia Repair will be clearly demonstrated, along with any associated organoax-
Unlike most operations on the esophagus, which are extirpative ial volvulus. A simple barium swallow often yields the most useful
procedures, hiatal hernia repair with fundoplication is a recon- information for managing the complex problem of recurrent hiatal
structive procedure, the aim of which is to restore a high-pressure hernia and a slipped Nissen fundoplication.
zone at the esophagogastric junction that prevents reflux but also Next, esophagogastroscopy should be performed to examine
permits comfortable swallowing. Currently, this repair is often the mucosa for the presence of esophagitis, Barrett’s mucosa, stric-
accomplished via minimally invasive approaches; however, such ture, or malignancy. The locations of any lesions observed, along
approaches may be hampered by significant perceptual and motor with the position of the squamocolumnar junction, should be care-
limitations, such as loss of stereopsis, reduced tactile feedback, and fully documented in terms of their distance from the incisors. All
decreased range of motion for the instruments.The degree of ten- strictures must undergo cup or brush biopsy to rule out an occult
sion on the hiatal repair sutures, the quality of the crural tissue malignancy.The presence of severe esophagitis raises the possibil-
itself, and the caliber of the esophageal hiatus after repair all must ity of acquired shortening of the esophagus secondary to trans-
be assessed. In certain patients, laparoscopic reconstruction of a mural inflammation and contraction scarring. Every effort should
competent gastroesophageal high-pressure zone may be very diffi- be made to measure the length of the esophagus accurately.
cult and may demand a degree of tactile sensitivity that is not yet
achievable via video laparoscopy. Dilation
The long-term success of antireflux surgery, whether done via If a stricture is found during esophagoscopy, a decision must be
the transthoracic approach or by means of laparoscopy, depends made about whether to attempt esophageal dilation. This proce-
on three factors: (1) a tension-free repair that maintains a 4 cm dure carries the risk of perforation and should be performed only
long segment of esophagus in the intra-abdominal position, (2) after careful consideration. If the stricture is diagnosed at the time
durable approximation of the diaphragmatic crura, and (3) correct of the initial endoscopic examination, it is advisable to perform
matching of the fundoplication technique chosen to the peristaltic only the brush biopsy at this point, deferring dilation to a subse-
function of the esophagus. The transthoracic approach should be quent visit. Delaying dilation gives the surgeon time to reassess the
considered whenever the standard abdominal approaches to hiatal anatomy depicted on the barium swallow, to decide whether wire-
hernia repair carry an increased risk of failure or complication— guided dilation is necessitated by angulation of the esophagus, to
for example, in patients who have a foreshortened esophagus asso- obtain informed consent, to assemble the requisite equipment,
ciated with a massive hernia and an incarcerated intrathoracic and to plan sedation for what is often an uncomfortable proce-
stomach, patients with severe peptic strictures of the esophagus, dure. If a malignancy is suspected at the time of the initial endo-
patients in whom the hiatal hernia coexists with an esophageal scopic examination, dilation should be avoided. In this situation,
motility disorder or morbid obesity, and patients who have under- repair is impossible; thus, if iatrogenic perforation of a malignant
gone multiple previous abdominal operations. The transthoracic stricture occurs, the surgeon will have to attempt emergency resec-
repair is particularly useful when a previous open abdominal pro- tion in an inadequately prepared patient in whom proper staging
cedure has failed. In this situation, the reasons for such failure, is unlikely to have been completed.
whether technical or tissue-related, should be assessed so that a The standard flexible adult esophagoscope is approximately 32
compensatory strategy can be devised. French in caliber. In advancing the scope into the stricture, only
very gentle pressure should be necessary. As a rule, a mild stricture
PREOPERATIVE EVALUATION
that is not associated with steep angulation of the esophagus will
readily accept passage of the endoscope and will be amenable to
Symptomatic Evaluation subsequent blind dilation with Hurst-Maloney bougies.
All patients being considered for fundoplication to treat GERD After successful passage, the scope is removed, and sequential
must undergo a comprehensive evaluation to determine whether insertion of progressively larger dilators (starting at 32 French) into
there is indeed an anatomic substrate for their symptoms and what the stricture is attempted.The weight of the dilator alone should be](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-4-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 5
sufficient to effect its passage, with little or no forward force performed by the operating team. Insufflation should be done
applied. Although the patient will be able to swallow comfortably with as little air as is practical, particularly in the case of large
only after satisfactory passage of a dilator at least 48 French in cal- paraesophageal hernias. The extent of the pathologic condition is
iber, it is essential never to try to force passage.To this end, the sur- documented and the absence of malignancy verified.The stomach
geon must take careful note of the subtle signs of increasing resis- is decompressed with suction, and the endoscope is removed. An
tance transmitted through the dilator. Sequential dilation should orogastric tube is placed while the patient is supine.
be stopped whenever significant resistance is encountered or blood Tracheal intubation is performed with either a standard single-
streaks appear on the dilator. Sudden pain during dilation is an lumen endotracheal tube or a double-lumen tube. The former
ominous sign and calls for immediate investigation with a swallow requires that the ventilated left lung be retracted cephalad with
study using a water-soluble contrast agent (e.g., Gastrografin; moist packs during the procedure; the latter allows lung isolation
Schering AG, Berlin, Germany). Subcutaneous emphysema in the and is preferred by some surgeons. A Foley catheter is placed; cen-
neck or mediastinal air on a plain chest radiograph may also indi- tral venous access generally is not required. Subcutaneous heparin
cate an injury to the esophagus. Perforation must be definitively is administered for DVT prophylaxis, and pneumatic calf com-
ruled out before the patient can be discharged. pression devices are applied. Antibiotic prophylaxis is provided
Highly stenotic strictures that do not allow the passage of a [see 1:1 Prevention of Postoperative Infection].
standard adult endoscope may be associated with a distorted and The patient is positioned for a left thoracotomy. The table is
a steeply angulated esophagus. In such cases, the use of a pediatric flexed to distract the ribs. An axillary roll is placed to protect the
endoscope may permit directed placement of a guide wire right brachial plexus. The right leg is bent at hip and knee while
through the stricture; fluoroscopy is a useful adjunct for this pur- the left leg is kept straight. Pillows are placed between the legs, and
pose. A series of progressively larger Savary-Gillard dilators may all pressure points are padded.The arms are positioned so that the
then be passed over the guide wire to enlarge the lumen and allow humeri are at right angles to the chest and the elbows are bent 90º.
subsequent endoscopic biopsy. As a rule, much less tactile feed-
OPERATIVE TECHNIQUE
back is available during wire-guided dilation than during passage
of standard Maloney-Hurst bougies. Increased pressure is
required to pass the Savary-Gillard dilators because of the resis- Step 1: Incision and Entry into Chest
tance caused by the wire passing through the dilator itself. It is A standard left posterolateral thoracotomy is performed. The
essential that the wire be well lubricated and not be allowed to dis- latissimus dorsi is divided. The serratus fascia is incised, but the
lodge proximally between the sequential insertions of progressive- muscle itself can generally be preserved. For most patients, the
ly larger dilators.The caveats that apply to blind dilation also apply sixth interspace is the most appropriate incision site for exposing
to wire-guided dilation. the hiatus.The seventh interspace can also be used, particularly if
Patients whose esophagus can be dilated to 48 French and who the patient is tall or has a hyperextended chest as a result of chron-
are candidates for antireflux surgery may undergo subsequent ic pulmonary disease. The paraspinal muscles are elevated away
intraoperative dilation to 54 to 60 French. Patients who cannot be from the posterior aspect of the adjacent ribs, and a 1 cm segment
dilated to 48 French and fail to achieve comfortable swallowing of the rib below the selected interspace is resected to facilitate
should be classified as having a non-dilatable stricture and should exposure. The chest is then entered, and the lung and the pleural
be considered for transhiatal esophagectomy [see Resection of space are thoroughly inspected. The leaves of the retractor are
Esophagus and Proximal Stomach, below]. spread slowly over the course of the next several minutes so as not
to cause iatrogenic rib fractures.
Functional Evaluation
Esophageal manometry permits quantitative assessment of Step 2: Mobilization of Esophagus and Excision of Hernia Sac
peristalsis, a capability that is critically important for determining The inferior pulmonary ligament is divided with the electro-
which type of fundoplication is most suitable for reconstructing a cautery to the level of the inferior pulmonary vein [see Figure 4].
nonoccluding high-pressure zone at the esophagogastric junction. The mediastinal pleura overlying the esophagus is longitudinally
Stationary pH tests measure the capacity of the esophagus to clear incised to expose the esophagus from the level of the carina to the
acid, its sensitivity to instilled acid, the relationship of reflux diaphragm. Particular care is taken to avoid injury to the vagi.
episodes to body position, and the correlation between changes in Vessels supplying the esophagus and arising from the adjacent
esophageal pH and the subjective symptoms of heartburn. aorta are cauterized and divided. A few larger vessels may have to
Ambulatory 24-hour pH testing allows further quantification of be ligated with 2-0 silk.The esophagus is encircled just below the
reflux episodes with respect to duration, frequency, and associa- inferior pulmonary vein with a wide Penrose drain [see Figure 4].
tion with patient symptoms. The two vagi are mobilized and carried with the esophagus. (The
right vagus is located along the right anterior border of the des-
OPERATIVE PLANNING
cending aorta and can easily be missed.)
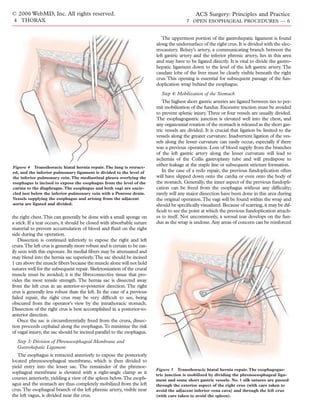
The transthoracic hiatus hernia repair may be completed with The esophagus is then elevated, and mobilization is circumfer-
either a partial fundoplication (as in the 240º Belsey Mark IV pro- entially completed in the direction of the diaphragm, starting from
cedure) or a complete fundoplication (as in the 360º Nissen pro- the level of the carina. In cases of giant paraesophageal hernia or
cedure). Acquired shortening of the esophagus may necessitate reoperation for a failed repair, the stomach will have a large intra-
lengthening of the esophagus by means of a Collis gastroplasty, in thoracic component. Dissection continues inferiorly to separate
which the portion of the gastric cardia along the lesser curvature the sac from the pericardium anteriorly and the aorta posteriorly.
and directly contiguous to the distal esophagus is fashioned into a The right pleura is closely approximated to the esophagus for 2 to
tube [see Operative Technique, Step 6a, below]. 5 cm above the diaphragm; in the presence of a substantial hiatal
A thoracic epidural catheter is placed for regional analgesia. hernia and its sac, it may be difficult to identify. The right pleura
General anesthesia is administered, and flexible esophagoscopy is should be gently dissected away from the sac without entry into](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-5-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 7
with a simple stitch of 4-0 silk. Mobilization is complete when the
fundus is restored to its original anatomic position and the greater
curvature can be followed down to the left gastroepiploic artery.
Step 5: Closure of Crura
Because the right crus is often quite attenuated, it is crucial to
incorporate an adequate amount of tissue into the repair. An Allis
or Babcock clamp is placed at the apex of the hiatus and into the
central tendon so that both crura can be placed under tension.
The esophagus is retracted anteriorly, and a No. 1 silk suture is
passed through the most posterior aspect of the left crus, with care
taken to avoid the adjacent spleen [see Figure 5]. A notched spoon
retractor is placed through the hiatus and into the abdomen
behind the left crus.The spleen is thus protected while the suture
is brought through the left crus.
Next, the suture is brought out through the right crus, with care
taken to prevent injury to the aorta or entry through the right
pleura.Three to five crural repair stitches are then placed at 1 cm
intervals, from posterior to anterior. The sutures should be stag-
gered slightly so that the needle entry points are not all in a
straight line; this measure helps prevent longitudinal shredding of
the muscle fibers when the sutures are placed under tension.The
sutures are held together with hemostats but left untied at this Figure 6 Transthoracic hiatal hernia repair. The anterior fat
point in the operation. pad is removed from the esophagus with sharp dissection, with
care taken to avoid injury to the vagi.
Placement of traction on the last suture should close the defect
while still allowing easy passage of one finger along the esophagus.
The final decision on whether to tie this last suture or to cut it out
is made later, after construction of the fundoplication. It is better Step 7: Fundoplication and Reduction of Wrap into Abdomen
to err on the side of an overly narrow opening: removing a suture The fundus is passed posteriorly behind the esophagus and
is easier than having to place an extra one at a time when expo- brought up against the anterior stomach, with care taken to avoid
sure is less than optimal. torsion of the fundal wrap.The fundus is then wrapped either over
the lower 2 cm of the esophagus, if no gastroplasty was done, or
Step 6: Assessment of Esophageal Length and Removal of over a 2 cm length of the gastroplasty tube while the bougie is in
Anterior Fat Pad place. The seromuscular layer of the fundus is approximated to
After placement of the crural stitches, an assessment of the that of the esophagus or the gastroplasty tube and that of the adja-
esophageal length is made. Ideally, the stomach can easily be cent anterior stomach with two interrupted 2-0 silk sutures [see
reduced into the abdomen without placing tension on the thoracic Figure 8]. When tied, the wrap should still be loose enough to
esophagus.When esophageal foreshortening is found, a Collis gas- accommodate a finger alongside the esophagus. The fundoplica-
troplasty is performed [see Step 6a, below]. tion sutures are again oversewn with a continuous seromuscular
If an esophageal stricture is present, the assistant performs dila- nonabsorbable monofilament suture. Two clips are placed at the
tion by passing a tapered bougie orally while the surgeon supports superior aspect of the wrap. These, along with the previously
the esophagus. The anteriorly located esophageal fat pad is placed clips, help confirm both the length and the location of the
removed in anticipation of the gastroplasty, with care taken not to wrap on chest x-ray.
injure the vagi located on either side [see Figure 6]. Once the fundoplication is complete, the dilator is removed and
the wrap is reduced into the abdomen. Two mattress sutures of
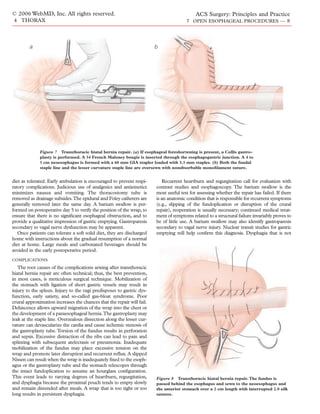
Step 6a: Collis Gastroplasty 2-0 polypropylene are placed to secure the top of the fundoplica-
In a Collis gastroplasty for a short esophagus, a stapler is used tion to the underside of the diaphragm. The crural sutures are
to form a 4 to 5 cm neoesophagus out of the proximal stomach, then sequentially tied, from the most posterior one to the most
thereby effectively lengthening the esophagus and transposing the anterior. When the final suture is tied, one finger should still be
esophagogastric junction more distally. A large-caliber Maloney able to pass through the hiatus alongside the esophagus.
bougie (54 French for women, 56 French for men) is placed in the
esophagus to prevent narrowing of the lumen as the stapler is Step 8: Drainage and Closure
fired. The bougie is advanced well into the stomach so that its A nasogastric tube is passed into the stomach and secured.
widest portion rests at the esophagogastric junction.The bougie is Hemostasis is verified, and a single thoracostomy tube is placed.
held against the lesser curvature, and the fundus is retracted away The wound is closed in layers. A chest x-ray is performed to veri-
at a right angle to the esophagus with a Babcock clamp. A 60 mm fy the position of the tubes and the location of the clips marking
gastrointestinal anastomosis (GIA) stapler loaded with 3.5 mm the wrap. The patient is then extubated in the OR and transport-
staples is applied immediately alongside the bougie on the greater ed to the recovery area.
curvature side [see Figure 7a] and fired, simultaneously cutting
POSTOPERATIVE CARE
and stapling the cardia. The staple line is oversewn with nonab-
sorbable 4-0 monofilament suture material on both sides [see Patients typically remain in the hospital for 5 days.The nasogas-
Figure 7b].Two metal clips are placed to mark the distal extent of tric tube is left on low suction and removed on postoperative day
the gastroplasty tube, denoting the new esophagogastric junction. 3. Patients then begin liquid oral intake, advancing to a full fluid](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-7-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 9
related to recurrent reflux, ulceration, or stricture usually responds become apparent only at the time of operation. Thoracic epidural
to dilation; reoperation is not required if the barium swallow shows analgesia should be administered for pain control. If an Ivor-Lewis
contrast flowing through the esophagus and an intact wrap beneath or thoracoabdominal approach is taken, a double-lumen endotra-
the diaphragm. Given that patients with longstanding reflux are at cheal tube should be placed for separate lung ventilation.
higher risk for dysplasia and esophageal adenocarcinoma, it is
important to perform endoscopy to rule out malignancy. Imaging
Contrast esophagography, esophagoscopy with biopsy, and
OUTCOME EVALUATION
contrast-enhanced CT of the chest and the upper abdomen are
Transthoracic hiatal hernia repair yields good to excellent required before esophagectomy. The esophagogram identifies the
results in more than 85% of patients undergoing a primary repair. location of the tumor and may indicate whether it extends into the
Approximately 75% of patients who have previously undergone proximal stomach. Esophagoscopy allows direct assessment of the
hiatal hernia repair experience symptomatic improvement.2 mucosa, precise localization of the tumor, and collection of tissue
for histologic study. Retroflexion views of the stomach, after dis-
tention with air, are particularly important if proximal gastric inva-
Resection of Esophagus and Proximal Stomach sion is suspected, in which case esophagogastrectomy with recon-
In the remainder of the chapter, we describe the standard open struction of alimentary continuity by means of intestinal interpo-
techniques for resection of the esophagus and the esophagogastric sition may be required. In cases of midesophageal cancer, a bron-
junction. Transhiatal esophagectomy is commonly performed to choscopy is mandatory to rule out airway involvement. The cari-
treat end-stage benign esophageal disease and carcinomas of the na and the proximal left mainstem bronchus are the sites that are
cardia and the lower esophagus. Esophageal resection through a most at risk for local invasion.
combined laparotomy–right thoracotomy approach is ideal for Contrast-enhanced CT scans of the chest and abdomen are
cancers of the middle and upper esophagus. The gastric conduit standard.Thoracic and abdominal CT scans yield information on
may be anastomosed to the cervical esophagus either high in the the extent of any celiac or mediastinal adenopathy, the degree of
right chest (as in an Ivor-Lewis esophagectomy) or in the neck (as esophageal thickening, and the possibility of invasion of the adja-
in a transhiatal esophagectomy).The left thoracoabdominal approach cent aorta or tracheobronchial tree. The lung parenchyma is
is rarely used but may be indicated for resection of the distal esoph- assessed for metastatic nodules, as are the liver and the adrenal
agus and the proximal stomach in the case of a bulky tumor that glands. When the distal extent of tumor cannot be defined as a
is locally aggressive. result of near-complete obstruction on endoscopy, a prone
abdominal CT can help differentiate a tumor at the gastric cardia
PREOPERATIVE EVALUATION
from a collapsed but normal stomach. If the obstruction is not
Thorough preoperative preparation is essential for good post- complete, the stomach can be distended with air (through either
operative outcome. Smoking cessation and a graded regimen of the ingestion of effervescent granules or the passage of a small-
home exercise will help minimize postoperative complications and bore nasogastric tube) to improve visualization. A prone CT also
encourage early mobilization. Schematic diagrams have proved yields improved imaging of the gastrohepatic and celiac lymph
useful for educating patients and shaping their expectations about nodes by allowing the stomach to fall away from these adjacent
quality of life and ability to swallow after esophagectomy. structures. Metastatic cancer in the celiac lymph nodes portends
Illustrations, by emphasizing the anatomic relations, greatly facili- a very poor prognosis and is a contraindication to resection.
tate discussion of potential complications (e.g., hoarseness from PET scanning is useful for the detection of occult distant
recurrent laryngeal nerve injury, pneumothorax, anastomotic metastases that preclude curative resection. Suspicious areas
leakage, mediastinal bleeding, and splenic injury). should undergo needle biopsy or laparoscopic or thoracoscopic
Potential postoperative problems (e.g., reflux, regurgitation, assessment. Similarly, pleural effusions [see 4:4 Pleural Effusion]
early satiety, dumping, and dysphagia) must be discussed before must be tapped for cytologic evaluation. Invasion of mediastinal
operation. Such discussion is particularly relevant for patients structures and the presence of distant metastases are contraindi-
undergoing esophagectomy for early-stage malignant tumors or cations to transhiatal esophagectomy.
for high-grade dysplasia in Barrett’s mucosa. These patients gen- At present, EUS, though quite sensitive for detection of parae-
erally have no esophageal obstruction and may be completely sophageal adenopathy, is incapable of differentiating reactive
asymptomatic; accordingly, their expectations about postoperative lymph nodes from nodes invaded by malignancy. CT and PET
function may be quite different from those of patients with pro- have limitations, and thus, locoregional involvement may not be
found dysphagia secondary to near-complete esophageal occlu- recognized before resection is attempted. In patients who are mar-
sion. Support groups in which patients with upcoming operations ginal candidates for surgical treatment and in whom metastatic
can contact patients that have already undergone treatment have disease is suspected, thoracoscopy and laparoscopy have been
proved to be highly beneficial to all parties. Realistic expectations advocated for histologic evaluation of mediastinal lymph nodes,
improve the chances of a satisfactory outcome. pleural or peritoneal abnormalities, and celiac nodes. Although
this approach adds to the cost of investigation, it can save the
Evaluation of Operative Risk patient from having to undergo a major operation for what would
Preoperative assessment should include a thorough review of the later prove to be an incurable condition.
patient’s cardiopulmonary reserve and an estimate of the level of
operative risk [see ECP:6 Risk Stratification, Preoperative Testing, and Neoadjuvant Therapy
Operative Planning]. Spirometry, arterial blood gas analysis, and Patients with esophageal cancer who are candidates for resec-
exercise stress testing should be considered. Even when a transhiatal tion may benefit from neoadjuvant chemotherapy and concurrent
esophagectomy without thoracotomy is planned, patients should be radiation therapy. In particular, patients with good performance
assessed with an eye to whether they can tolerate a laparotomy and status and bulky disease should be considered for such therapy.To
a thoracotomy, in case the latter is made necessary by findings that date, no randomized trials have conclusively demonstrated a sur-](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-9-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 10
vival benefit with this approach, but several series have document- Flexible esophagoscopy is performed (if it was not previously per-
ed a 20% to 30% rate of complete response with no viable tumor formed by the surgical team). A nasogastric tube is placed before
found at the time of resection. After chemoradiation, patients are final positioning and draping.
restaged with a barium swallow and CT. PET scanning after treat- The patient is placed in the supine position with a small rolled
ment may yield spurious results, in that inflammatory conditions sheet between the shoulders. The arms are secured to the sides,
can mimic the increased tracer uptake seen in malignant tissue. and the head is rotated to the right with the neck extended. The
Microscopic disease cannot be assessed, and scarring from radia- neck, the chest, and the abdomen are prepared as a single sterile
tion may further confound the situation by preventing tracer field. The drapes are placed so as to expose the patient from the
uptake in areas that actually harbor malignancy. left ear to the pubis.The operative field is extended laterally to the
If there are no contraindications to surgical treatment, resection anterior axillary lines to permit placement of thoracostomy tubes
is scheduled 2 to 3 weeks after the completion of neoadjuvant as needed. A self-retaining table-mounted retractor is used to facil-
therapy. This interval allows time for patients to return to their itate upward and lateral traction along the costal margin.
baseline activity level and for any induced hematologic abnormal-
ities to be corrected. Previous chemoradiation therapy does not Ivor-Lewis Esophagectomy
make transhiatal esophagectomy significantly more difficult or At many institutions, Ivor-Lewis esophagectomy is preferred
complicated. Many tumors are downstaged and less bulky at the because it provides excellent direct exposure for dissection of the
time of resection. In centers with experience in this approach, the intrathoracic esophagus, in that it combines a right thoracotomy
rates of bleeding and anastomotic leakage remain low. with a laparotomy. This procedure should be considered when
there is concern regarding the extent of esophageal fixation with-
OPERATIVE PLANNING
in the mediastinum. One advantage of Ivor-Lewis esophagectomy
is that an extensive local lymphadenectomy can easily be per-
Transhiatal Esophagectomy formed through the right thoracotomy. Any attachments to medi-
In transhiatal esophagectomy, the stomach is mobilized through astinal structures can be freed under direct vision. Whether any
a short upper midline laparotomy, the esophagus is mobilized regional lymph node dissection is necessary is highly controversial;
from adjacent mediastinal structures via dissection through the no significant survival advantage has yet been demonstrated.
hiatus without the use of a thoracotomy, and the stomach is trans- Long-term survival after Ivor-Lewis resection is equivalent to that
posed through the posterior mediastinum and anastomosed to the after transhiatal esophagectomy.3
cervical esophagus at the level of the clavicles. The main advan- The main disadvantages of the Ivor-Lewis procedure are (1) the
tages of this approach are (1) a proximal surgical margin that is physiologic impact of the two major access incisions employed (a
well away from the tumor site, (2) an extrathoracic esophagogas- right thoracotomy and a midline laparotomy) and (2) the location
tric anastomosis that is easily accessible in the event of complica- of the anastomosis (in the chest, at the level of the azygos vein).
tions, and (3) reduced overall operative trauma. Single-center stud- Incision-related pain may hinder deep breathing and the clearing
ies throughout the world have shown transhiatal esophagectomy to of bronchial secretions, resulting in atelectasis and pneumonia.
be safe and well tolerated, even in patients who may have signifi- Complications of the intrathoracic anastomosis may be hard to
cantly reduced cardiopulmonary reserve. Long-term survival is manage. Although the anastomotic leakage rate associated with
equivalent to that reported after transthoracic esophagectomy. Ivor-Lewis esophagectomy has typically been 5% or lower—and
Although transhiatal esophagectomy has been used for resec- thus substantially lower than the rate cited for the cervical anasto-
tion of tumors at any location in the esophagus, it is best suited for mosis after transhiatal esophagectomy—intrathoracic leaks are
resection of tumors in the lower esophagus and at the esopha- much more dangerous and difficult to handle than intracervial
gogastric junction. It should also be considered the operation of leaks. In many cases, drainage of the leak will be incomplete and
choice for certain advanced nonmalignant conditions of the empyema will result. Reoperation may prove necessary to manage
esophagus. Nondilatable strictures of the esophagus may occur as mediastinitis.
an end-stage complication of gastroesophageal reflux. Intractable
reflux after failed hiatal hernia repair may not be amenable to fur- Left Thoracoabdominal Esophagogastrectomy
ther attempts at reconstruction of the esophagogastric junction The left thoracoabdominal approach is indicated for resection
and thus may call for esophagectomy. Because of the high cervical of the distal esophagus and the proximal stomach when removal
anastomosis, a transhiatal esophagectomy is less likely to predis- of the stomach necessitates the use of an intestinal substitute to
pose to postoperative reflux and recurrent stricture formation than restore swallowing. If the proximal stomach must be resected for
a transthoracic esophagectomy would be. Achalasia may result in adequate resection margins to be obtained, then the distal stom-
a sigmoid megaesophagus and dysphagia that cannot be managed ach may be anastomosed to the esophagus in the chest.This oper-
without removal of the esophagus. Transhiatal esophagectomy ation is frequently associated with significant esophagitis from bile
permits complete removal of the thoracic esophagus and, in the reflux, and dysphagia is common. Consequently, many surgeons
majority of patients, restoration of comfortable swallowing with- prefer to resect the entire stomach and the distal esophagus and
out the need for a thoracotomy. then to restore swallowing with a Roux-en-Y jejunal interposition
Generally, patients are admitted to the hospital on the day of the anastomosed to the residual thoracic esophagus.
operation.Thoracic epidural analgesia is administered, both intra-
OPERATIVE TECHNIQUE
operatively and postoperatively, and appropriate antibiotic pro-
phylaxis is provided [see 1:1 Prevention of Postoperative Infection].
Heparin, 5,000 U subcutaneously, is given before induction, and Transhiatal Esophagectomy
pneumatic calf compression devices are applied. A radial artery Transhiatal esophagectomy is best understood as consisting of
catheter is placed to permit continuous monitoring of blood pres- three components: abdominal, mediastinal, and cervical. The ab-
sure. Central venous access is rarely required. General anesthesia dominal portion involves mobilization of the stomach, pyloromy-
is administered via an uncut single-lumen endotracheal tube. otomy, and placement of a temporary feeding jejunostomy.](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-10-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 11
Step 1: incision and entry into peritoneum A midline Dissection then proceeds along the greater curvature toward
laparotomy is performed from the tip of the xiphoid to the umbili- the pylorus. The omentum is mobilized from the right gastroepi-
cus.The peritoneum is opened to the left of the midline so that the ploic artery.Vessels are ligated between 2-0 silk ties, and great care
falciform and the preperitoneal fat may be retracted en bloc to the is exercised to avoid placing excessive traction on the arterial
right. Body wall retractors are placed at 45º angles from the mid- arcade. A 1 cm margin is always maintained between the line of
line to elevate and distract both costal margins.The retractors are dissection and the right gastroepiploic artery. Venous injuries, in
placed so as to lift up the costal margin gently and open the particular, can occur with injudicious handling of tissue. The
wound. The abdomen is then inspected for metastases. ultrasonic scalpel is particularly efficient and effective for mobi-
lization of the stomach; again, this instrument must be applied
Step 2: division of gastrohepatic ligament and mobiliza- well away from the gastroepiploic arcade. Dissection is continued
tion of distal esophagus The left lobe of the liver is mobilized rightward to the level of the pylorus. It should be noted that the
by dividing the triangular ligament, then folded to the right and location of the gastroepiploic artery in this area may vary; often, it
held in this position with a moist laparotomy pad and a deep-blad- is at some unexpected distance from the stomach wall. Posterior
ed self-retaining retractor. Next, the gastrohepatic ligament is divided. adhesions between the stomach and the pancreas are lysed so that
Occasionally, there is an aberrant left hepatic artery arising from the lesser sac can be completely opened.
the left gastric artery [see Figure 9].The peritoneum over the right The assistant’s left hand is then placed into the lesser sac to
crus is incised, and the hiatus is palpated; the extent and mobility retract the stomach gently to the right and place the short gastric
of any tumor may then be assessed. The peritoneum over the left vessels on tension. The Penrose drain previously placed around
crus is similarly divided, and the esophagus is encircled with a 2.5 cm the esophagus facilitates exposure by retracting the cardia to the
Penrose drain. Traction is applied to draw the esophagogastric right. Dissection along the greater curvature proceeds cephalad.
junction upward and to the right; this measure facilitates exposure The vessels are divided well away from the wall of the stomach to
of the short gastric arteries coursing to the fundus and the cardia. prevent injury to the fundus. Clamps should never be placed on
the stomach. A high short gastric artery is typically encountered
Step 3: mobilization of stomach The greater curvature of just adjacent to the left crus. Precise technique is required to pre-
the stomach is inspected and the right gastroepiploic artery pal- vent injury to the spleen. The Penrose drain [see Step 2, above] is
pated.The lesser sac is generally entered near the midpoint of the exposed as the peritoneum is opened over the left crus.
greater curvature. The transition zone between the right gas- Mobilization of the proximal stomach and liberation of the distal
troepiploic arcade and the short gastric arteries is usually devoid esophagus are thereby completed.
of blood vessels. A moist sponge is placed behind the spleen to ele- Once the stomach has been completely mobilized along the
vate it and facilitate subsequent control of the short gastric vessels. greater curvature, it is elevated and rotated to the right [see Figure
Divided Left
Gastric Vessels
Lesser Omentum
Divided
Figure 9 Transhiatal esophagec- Greater Omentum
tomy. The duodenum is mobilized, Divided
and the gastrohepatic and gastro-
colic omenta are divided.
Intact Right
Gastroepiploic
Vessels](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-11-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 12
Hepatic Splenic
Artery Artery
E
Intact Right
Gastroepiploic
Artery
Figure 10 Transhiatal esophagectomy. After the stomach has been completely mobilized A
along the greater curvature, it is elevated and rotated to the right. The left gastric vessels
are suture-ligated and divided. A 1 cm margin of the diaphragmatic crura is taken in con-
tinuity with the esophagogastric junction, providing ample clearance of the tumor and
improved exposure of the lower mediastinum.
10]; the left gastric artery and associated nodal tissues can then be Dissection is extended toward the duodenum with the aid of a
visualized via the lesser sac. The superior edge of the pancreas is fine-tipped right-angle clamp. The duodenal submucosa, recog-
visible, and the remaining posterior attachments of the stomach nizable by its fatty deposits and yellow coloration, is exposed for
are divided along the hiatus and the left crus.These may be quite approximately 0.5 cm. The duodenal submucosa is usually much
extensive if there has been a history of pancreatitis or preoperative more superficial than expected, and accidental entry into the duo-
radiation therapy. denum often occurs just past the left edge of the circular muscle
If the operation is being done for malignant disease, a final of the pylorus. Releasing the tension on the traction sutures helps
determination of resectability can be made at this point. Tumor the surgeon visualize the proper depth of dissection. Should entry
fixation to the aorta or the retroperitoneum can be assessed. Celiac into the lumen occur, a simple repair using interrupted fine
and paraortic lymph nodes can be palpated and, if necessary, sent monofilament (4-0 or 5-0 polypropylene) sutures to close the
for biopsy. The left gastric artery and vein are then ligated proxi- mucosa is performed. Small metal clips are applied to the knots of
mally, either through the lesser sac or directly through the divided the traction sutures before removal of the ends; these clips serve
gastrohepatic ligament. All nodal tissue is dissected free in antici- to indicate the level of the pyloromyotomy on subsequent radi-
pation of subsequent removal en bloc with the specimen. ographic studies.
Step 4: mobilization of duodenum and pyloromyotomy Step 5: feeding jejunostomy Placement of a standard
The duodenum is mobilized with a Kocher maneuver. Careful Weitzel jejunal feeding tube approximately 30 cm from the liga-
attention to the superior extent of this dissection is critical. ment of Treitz completes the abdominal portion of the transhiatal
Adhesions to either the porta hepatis or the gallbladder must be esophagectomy.
divided to ensure that the pylorus is sufficiently freed for later
migration to the diaphragmatic hiatus. Step 6: exposure and encirclement of cervical esophagus
Gastric drainage is provided by a pyloromyotomy. Two figure- The cervical esophagus is exposed through a 6 cm incision along
eight traction sutures of 2-0 cardiovascular silk are placed deeply the anterior edge of the left sternocleidomastoid muscle [see Figure
through both the superior and the inferior border of the pylorus; 1] that is centered over the level of the cricoid cartilage. The
traction is then placed on these sutures to provide both exposure platysma is divided to expose the omohyoid, which is divided at its
and some degree of hemostasis.The pyloromyotomy is begun 2 to tendon.The strap muscles are divided low in the neck.The esoph-
3 cm on the gastric side of the pylorus. The serosa and the mus- agus and its indwelling nasogastric tube can be palpated.
cle are divided with a needle-tipped electrocautery to expose the The carotid sheath is retracted laterally, and blunt dissection is
submucosa; generally, these layers of the stomach are robust, mak- employed to reach the prevertebral fascia. The inferior thyroid
ing the proper plane easy to find. artery is ligated laterally; the recurrent laryngeal nerve is visible just](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-12-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 13
deep and medial to this vessel. No retractor other than the surgeon’s
finger should be applied medially: traction injury to the recurrent
laryngeal nerve will result in both vocal cord palsy and uncoordi-
nated swallowing with aspiration. In particular, metal retractors
must not be used in this area. The tracheoesophageal groove is
incised close to the esophageal wall while gentle finger traction is
applied cephalad to elevate the thyroid cartilage toward the right.
This measure usually suffices to define the location of the nerve.
The esophagus is then encircled by passing a right-angle clamp
posteriorly from left to right while the surgeon’s finger remains in
the tracheoesophageal groove.The tip of the clamp is brought into
the pulp of the fingertip.The medially located recurrent laryngeal
nerve and the membranous trachea are thereby protected from
injury.The clamp is brought around, and a narrow Penrose drain
is passed around the esophagus [see Figure 11]. Blunt finger dis-
section is employed to develop the anterior and posterior planes
around the esophagus at the level of the thoracic inlet.
Step 7: mediastinal dissection Some authors describe this
portion of the procedure as a blunt dissection, but in fact, the vast
majority of the mediastinal mobilization is done under direct
vision. Narrow, long-handled, handheld, curved Harrington
retractors are placed into the hiatus and lifted up to expose the
distal esophagus. Caudal traction is placed on the esophagus,
allowing excellent visualization of the hiatus and the distal esoph-
agus. Long right-angle clamps are used to expose these attach-
ments.Vascularity in this area is often minimal, and hemostasis can
easily be achieved with either the electrocautery or the ultrasonic
Cricopharyngeal Figure 12 Transhiatal esophagectomy. The plane posterior to the
Muscle esophagus is developed by placing the surgeon’s right hand into
the hiatus along the prevertebral fascia. A moist sponge stick is
placed through the cervical incision posterior to the esophagus,
and the posterior plane is completed.
scalpel. The left crus can be divided to facilitate exposure. Para-
esophageal lymph nodes are removed either en bloc or as separate
specimens. Dissection is continued cephalad with the electro-
cautery and a long-handled right-angle clamp. The two vagi are
divided, and the periesophageal adhesions are lysed. Mobilization
of the distal esophagus under direct vision is thus completed up to
the level of the carina.
Three specific maneuvers are now carried out. First, the plane
posterior to the esophagus is developed [see Figure 12]. The sur-
geon’s right hand is advanced palm upward into the hiatus, with
the fingers closely applied to the esophagus. The volar aspects of
the fingers run along the prevertebral fascia, elevating the esoph-
agus off the spine. A moist sponge stick is placed through the cer-
vical incision, also posterior to the esophagus. The sponge is
Left Recurrent advanced toward the right hand, which is positioned within the
Laryngeal Nerve mediastinum. As the sponge is advanced into the right palm, the
posterior plane is completed. A 28 French mediastinal sump is
then passed from the cervical incision into the abdomen along the
posterior esophageal wall and attached to suction. Any blood loss
from the mediastinum is collected and monitored.
Figure 11 Transhiatal esophagectomy. Once the cervical esoph-
Second, the anterior plane is developed [see Figure 13]. This is
agus is exposed through an incision along the left sternocleido- often much more difficult than developing the posterior plane
mastoid muscle, strap muscles are divided and retracted, and the because the left mainstem bronchus may be quite close to the
cervical esophagus is dissected away from the left and right esophagus. Again, the surgeon’s right hand is placed through the
recurrent laryngeal nerves. hiatus, but it is now palm down and anterior to the esophagus.The](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-13-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 14
fingertips enter the space between the esophagus and the left main- Pericardium Vagus Nerves
stem bronchus.The hand is gently advanced, and the airway is dis- Carina Clipped
placed anteriorly. A blunt curved suction handle is employed from
above as a substitute finger. It is advanced along the anterior aspect
of the esophagus through the cervical incision. The right hand
guides the tip of the suction handle beneath the bronchus. Lateral
displacement of the handle allows further mobilization of the
bronchus away from the esophagus. Completion of the anterior
and posterior planes usually results in a highly mobile esophagus.
Third, the lateral attachments of the upper and middle esopha-
gus are divided. Upward traction is applied with the Penrose drain
previously placed around the cervical esophagus, allowing further
dissection at the level of the thoracic inlet. Lateral attachments are
pushed caudally into the mediastinum, and traction applied to the
esophagus from below allows these attachments to be visualized
inferiorly through the hiatus, then isolated with long right-angle
clamps and divided with the electrocautery. Caution must be exer-
cised so as not to injure the azygos vein. Dissection on the right
side must therefore be kept close to the esophagus. Once the last
lateral attachment is divided, the esophagus is completely free and
can be advanced into the cervical wound.
Close monitoring of arterial blood pressure is maintained
throughout.Transient hypotension may occur as a result of medi-
astinal compression and temporary impairment of cardiac venous
return as the surgeon’s hand or retractors are passed through the
hiatus. Vasopressors are never required for management: simple
Figure 14 Transhiatal esophagectomy. The esophagus is divided
in the neck and delivered into the abdomen. Retractors are
placed in the hiatus, and any vessels entering into the esophagus
are clipped and divided. The vagi are also clipped and divided.
repositioning of the retractors or removal of the dissecting hand
usually results in prompt restoration of normal BP. Placement of
the patient in a slight Trendelenburg position is often helpful.
Step 8: proximal transection of esophagus and delivery
into abdomen The nasogastric tube is retracted to the level of
the cricopharyngeus, and the esophagus is divided with a cutting
stapler 5 to 6 cm distal to the muscle. The esophagus is then
removed via the abdomen [see Figure 14]. Retractors are placed in
the hiatus, and the mediastinum is inspected for hemostasis. The
sump is removed. Both pleurae are inspected.The lungs are inflat-
ed so that it can be determined which pleural space requires tho-
racostomy drainage. The mediastinum is packed with dry lapa-
rotomy pads from below. A narrow pack is placed into the thoracic
inlet from above. Chest tubes are then placed as required along the
inframammary crease in the anterior axillary line. The drainage
from these tubes should be closely monitored throughout the rest
of the operation to ensure that any bleeding from the mediastinal
dissection does not go unnoticed.
Step 9: excision of specimen and formation of gastric
tube The gastric fundus is grasped, and gentle tension is applied
along the length of the stomach. The esophagus is held at right
angles to the body of the stomach, and the fat in the gastrohepat-
ic ligament is elevated off the lesser curvature; all lymph nodes are
Figure 13 Transhiatal esophagectomy. The anterior plane is
thus mobilized. A point approximately midway along the lesser
developed by placing the surgeon’s right hand through the hiatus
anterior to the esophagus. The fingertips enter the space between
curvature is selected. The blood vessels traversing this area from
the esophagus and the left mainstem bronchus, to be met by a the right gastric artery are ligated to expose the lesser curvature.
blunt suction handle passed downward through the cervical inci- The distal resection margin is then marked; it should be 4 to 6 cm
sion. The lateral attachments of the esophagus are divided from from the esophagogastric junction, extending from the selected
above downward as far as the aortic arch. point on the lesser curvature to a point medial to the fundus. A 60](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-14-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 15
mm GIA stapler loaded with 3.5 mm staples is then used to tran- ensure that injury to the gastroepiploic arcade does not occur at
sect the proximal stomach, proceeding from the lesser curvature this late point in the procedure.The hiatus is narrowed, but not so
toward the fundus [see Figure 15]. Resection of the cardia along much that three fingers cannot be easily passed alongside the gas-
with the adjacent portion of the lesser curvature effectively con- tric conduit. This reconstitution will help prevent herniation of
verts a J-shaped stomach into a straight tube. other abdominal contents alongside the gastric conduit. The liver
For maximizing the length of the gastric tube, there are several is returned to its anatomic position, thus also preventing any sub-
technical points that are critical. Tension must be maintained on sequent herniation of bowel into the chest.The pyloromyotomy is
the stomach as the stapler is serially applied cephalad.The stapler generally found at the level of the diaphragm. The laparotomy is
should be simply placed on the stomach and fired: no attempt then closed in the usual fashion.The viability of the fundus in the
should be made to telescope tissue into the jaws, because to do so neck incision is checked periodically as the abdominal portion of
would effectively reconstitute the curve of the stomach and dimin- the procedure is completed.
ish its upward reach. Typically, three staple loads are required.
The specimen is removed, and frozen section examination is Step 11: cervical esophagogastric anastomosis The con-
done on the distal margin.The completed staple line is then over- struction of the esophagogastric anastomosis is the most impor-
sewn with a continuous Lembert suture of 4-0 polypropylene. Once tant part of the entire operation: any anastomotic complication
again, tension is maintained along the stomach to prevent any fore- will greatly compromise the patient’s ability to swallow comfort-
shortening of the lesser curvature.The use of two separate sutures, ably. Accordingly, meticulous technique is essential.
each reinforcing half of the staple line, is helpful in this regard. A seromuscular traction suture of 4-0 polyglactin is placed
through the anterior stomach at the level of the clavicle and drawn
Step 10: advancement of stomach into chest or neck upward, thus elevating the fundus into the neck wound and great-
The mediastinal packs are removed, and hemostasis is verified in ly facilitating the anastomosis.The pack behind the fundus is then
the chest.The stomach is inspected as well.The ends of any short removed.
gastric vessels that were divided with the ultrasonic scalpel are The site of the anterior gastrotomy is then carefully selected: it
now tied so that subsequent manipulation does not precipitate should be midway between the oversewn lesser curvature staple
bleeding. The stomach is oriented so that the greater curvature is line and the greater curvature of the fundus (marked by the ligat-
to the patient’s left.There must be no torsion.The anterior surface ed ends of the short gastric vessels). The staple line on the cervi-
of the fundal tip should be marked with ink so that proper orien- cal esophagus is removed, and the anterior aspect of the esopha-
tation of the stomach can be confirmed after its passage into the gus is grasped with a fine-toothed forceps at the level of the
neck. The stomach can usually be advanced through the posteri- planned gastrotomy. A straight DeBakey forceps is then applied
or mediastinum without any traction sutures or clamps. The sur- across the full width of the esophagus to act as a guide for division.
geon’s hand is placed palm down on the anterior surface of the The esophagus is cut with a new scalpel blade at a 45º angle so
stomach, with the fingertips about 5 cm proximal to the tip of the that the anterior wall is slightly longer than the posterior wall; the
fundus. The hand is then gently advanced through the chest,
pushing the stomach ahead of itself. The tip of the fundus is gen-
tly grasped with a Babcock clamp as it appears in the neck.To pre-
vent trauma at this most distant aspect of the gastric tube, the
clamp should not be ratcheted closed.
No attempt should be made to pull the stomach up into the
neck: the position of the fundus is simply maintained as the sur-
geon’s hand is removed from the mediastinum. Further length in
the neck can usually be gained by gently readvancing the hand
along the anterior aspect of the stomach.This measure uniformly
distributes tension along the tube and ensures proper torsion-free
orientation in the chest. The stomach is pushed up into the neck
rather than drawn up by the clamp.
A useful alternative approach for positioning the gastric tube
involves passing a large-bore Foley catheter through the medi-
astinum from the neck incision. The balloon is inflated, and a 50
cm section of a narrow plastic laparoscopic camera bag is tied
onto the catheter just above the balloon. The gastric tube is posi-
tioned within the bag, and suction is applied through the catheter,
creating an atraumatic seal between the stomach and the sur-
rounding plastic bag. As the bag is drawn upward through the
neck with gentle traction on the Foley catheter, the stomach
advances through the mediastinum. A small dry pack is placed in
the neck behind the fundus to prevent retraction into the chest.
The stomach is not secured to the prevertebral fascia in any way.
The feeding jejunal tube is brought out the left midabdomen
Figure 15 Transhiatal esophagectomy. A gastric tube is formed
through a separate stab incision.The hiatus is inspected for hemo- by stapling along the lesser curvature of the stomach from the
stasis, as is the splenic hilum. It may be necessary to reconstitute junction of the right and left gastric vessels to the top of the fun-
the hiatus with one or two simple sutures of 1-0 silk placed dus. This staple line is oversewn with a continuous 3-0 suture,
through the crura. These sutures must be placed with care to with care taken not to foreshorten the gastric tube.](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-15-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 16
anterior wall then forms the hood of the anastomosis. The fine- a
toothed forceps is used to maintain orientation of the esophagus
throughout. Two full-thickness stay sutures of 4-0 Vicryl are
placed, one at the midpoint of the anterior cut edge of the esoph-
agus and one at the corresponding location posteriorly.The poste-
rior stitch is placed from inside the lumen, and the needle is left
on the suture for later use.
A 2 cm gastrotomy is then performed with a needle-tipped elec-
trocautery using cutting current.The incision is obliquely oriented,
with the cephalad extent proceeding slightly medially. The needle
from the stay suture previously placed on the posterior wall of the
esophagus is then passed the full thickness of the cephalad aspect
of the gastrotomy [see Figure 16a]. Traction on this untied suture
brings the esophagus toward the stomach. A 45 mm endoscopic
stapler loaded with 3.5 mm staples is used to form the back wall of
the anastomosis.The thicker portion of the device (the cartridge) is
advanced cephalad into the esophagus, with the narrower portion
(the anvil) in the gastric lumen [see Figure 16b]. The tip of the sta-
pler should be aimed toward the patient’s right ear. Tension is
applied to the stay suture holding the esophagus and stomach
together so as to bring tissue into the jaws of the device. The por-
tion of the fundus extending beyond the stapler is then rotated
medially to ensure that the new staple line is well away from the one
previously placed along the lesser curvature.This is a crucial point: b
crossing of the two staple lines may create an ischemic area that can
give rise to a large leak in the postoperative period.
The stapler is then closed, holding the esophagus and stomach
together, but not yet fired. The position of the nasogastric tube
should be maintained just at the level of the cricopharyngeus dur-
ing the construction of the anastomosis.This positioning keeps the
tube out of the operative field and protects it from being entrapped
by the jaws of the stapler; it also facilitates subsequent passage of
the tube into the gastric conduit once the posterior wall of the
anastomosis is complete. Two suspension sutures are placed on
either side of the closed stapler, one toward the tip and the other
near the heel of the jaws.These four sutures alleviate any potential
tension on the staple line by approximating the muscular layer of
the esophagus to the seromuscular layer of the stomach. The sus-
pension sutures are tied, and the stapler is fired, thereby complet- c
ing the posterior portion of the anastomosis [see Figure 16c].
The anterior portion of the anastomosis is closed in two layers.
The inner layer consists of a continuous 4-0 polydioxanone suture
placed as full-thickness inverting stitches, and the second layer
consists of interrupted seromuscular Lembert sutures [see Figure
17]. The lateral and medial corners of the anastomosis, where the
staple line meets the handsewn portion, merit extra attention.
These corners are quite fragile, and excessive traction may result
in dehiscence progressing cephalad along the staple line in a zip-
perlike fashion.The inner layer should therefore be started at each Figure 16 Transhiatal esophagectomy. (a) After the proximal
corner, incorporating the last 5 mm of the staple line. Once sever- end of the stomach tube is delivered into the neck, the esophagus
al stitches have been placed from the two corners, the nasogastric is cut at a 45° angle so that the anterior wall is longer than the
tube can be passed through the anastomosis.The nasogastric tube posterior wall. A gastrotomy is placed between the oversewn less-
is properly positioned when the most distal black marker is at the er curvature staple line and the greater curvature of the fundus.
nares.The inner layer is then completed as the two sutures are tied A full-thickness suture is placed through all layers of the esopha-
at the midpoint. gus and all layers of the gastrotomy. (b) An endoscopic GIA sta-
pler is used to form the back wall of the anastomosis. The thicker
Step 12: drainage, closure, and completion x-ray A portion of the device (the cartridge) is advanced cephalad into
the esophagus, with the narrower portion (the anvil) in the gas-
small Penrose drain is placed in the thoracic inlet below the
tric lumen. The tip of the stapler should be aimed toward the
anastomosis and brought out through the inferior end of the patient’s right ear. The staple line must be well away from the
neck incision. The drain is secured, and the incision is irrigated. lesser curvature staple line. Two suspension sutures are placed on
The strap muscles are not reapproximated but are merely either side of the closed stapler, one toward the tip and the other
attached loosely to the underside of the sternocleidomastoid near the heel of the jaws. (c) The stapler is fired to complete the
muscle with two interrupted 4-0 polyglactin sutures. The platys- posterior portion of the anastomosis.](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-16-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 17
a Ivor-Lewis Esophagectomy
Steps 1 through 5 Esophagoscopy is performed to confirm
the location of the tumor. Steps 1, 2, 3, 4, and 5 of an Ivor-Lewis
esophagectomy are virtually identical to the first five steps (i.e., the
abdominal portion) of a transhiatal esophagectomy. Once com-
plete mobilization of the stomach is verified, the pylorus is manu-
ally advanced to the level of the diaphragm to ensure that it is not
being tethered by the duodenum or the greater omentum. The
stomach is then placed back into the anatomic position, and the
laparotomy is closed.
Step 6: exposure and mobilization of esophagus The
patient is shifted to the left lateral decubitus position and re-
draped. Single-lung ventilation is instituted, and the chest is
b entered through a right fifth or sixth interspace thoracotomy.The
inferior pulmonary ligament is divided, and the lung is retracted
cephalad. The esophagus is mobilized from the level of the dia-
phragm to a point above the azygos vein [see Figure 18], which is
typically divided with a vascular stapler. The pleura overlying the
esophagus is divided to the level of the thoracic inlet, superior to
the azygos vein. The esophagus is encircled with a Penrose drain
in the retrotracheal region. The pleura is then divided to the level
of the diaphragm, with care taken to stay close to the right bron-
chus and the pericardium and avoid injury to the thoracic duct.
Figure 17 Transhiatal esophagectomy. The anterior portion of
The soft tissue between the esophagus and the aorta posteriorly
the anastomosis is completed with (a) an inner layer consisting of and between the esophagus and the trachea or the pericardium
a continuous 4-0 polydioxanone suture and (b) an outer layer anteriorly is dissected free and maintained en bloc with the esoph-
consisting of interrupted sutures. agus. Periesophageal and subcarinal nodes are thereby mobilized
[see Figure 18b].
ma is reconstituted with interrupted 4-0 polyglactin sutures.The Step 7: excision and removal of specimen The hiatus is
nasogastric tube is secured. incised and the abdomen entered. The stomach is drawn up into
A chest x-ray is obtained in the OR to verify the position of the the chest, with care taken not to place excessive traction on the
drains and the absence of any abnormal collections in the chest. gastroepiploic pedicle. The esophagus is divided with a stapler
Patients are extubated in the OR and transported to the anesthet- proximally at least 5 cm away from any grossly evident tumor. A
ic recovery area. Extubation should be carried out only when the margin is sent for frozen section examination.The distal resection
health care team is confident that subsequent reintubation is margin is completed in a similar manner, and the esophageal
unlikely to be necessary. Emergency reintubation after a cervical specimen is removed from the operative field [see Figure 19]. The
anastomosis is hazardous, in that vigorous neck extension may gastric staple line is oversewn, and the stomach is positioned in the
threaten the suture line. Once patients are awake and alert, which posterior mediastinum.
is usually 3 to 4 hours after the operation, they are taken to the
general ward. As a rule, admission to an intensive care unit is not Step 8: intrathoracic esophagogastric anastomosis The
required unless there are substantial comorbidities or intraopera- site of the esophagogastric anastomosis should be about 2 cm
tive concerns. To prevent excessive traction on the anastomosis, above the divided azygos vein. Several interrupted sutures are
the neck should be maintained in a flexed position with two pil- used to secure the transposed stomach to the adjacent pleura.The
lows placed behind the head. staple line on the esophagus is removed, and a gastrotomy is per-
In certain patients, a stapled anastomosis may be impractical. formed in preparation for a side-to-side functional end-to-end
In patients with a bull-neck habitus, for example, a partial sternal anastomosis [see Figure 20].With the aid of full-thickness traction
split may be required for adequate exposure of the cervical esoph- sutures, the esophagus is positioned along the surface of the stom-
agus, and a handsewn true end-to-end anastomosis may be nec- ach and well away from the oversewn staple line defining the gas-
essary. In addition, patients who have previously undergone tric resection margin. The posterior aspect of the anastomosis is
antireflux surgery may have a relatively short gastric tube that will completed with an endoscopic GIA stapler as described earlier
necessitate an end-to-end reconstruction. [seeTranshiatal Esophagectomy, Step 11, above, and Figures 16 and
Patients should, if possible, begin walking the morning after the 17]. A nasogastric tube is passed, and the anterior wall is com-
operation. An incentive spirometer should be constantly within pleted in two layers.The first layer consists of a full-thickness con-
arm’s length of the patient, and hourly use of this device should tinuous 3-0 polydioxanone suture; the second consists of inter-
be encouraged.The nasogastric tube is removed on postoperative rupted absorbable sutures approximating the seromuscular layer
day 3, and the patient is allowed ice chips in the mouth.The tho- of the stomach to the muscular layer of the esophagus.
racic epidural catheter is removed the afternoon after the chest Two alternative methods of anastomosis are sometimes used:
tube is removed.The diet is gradually advanced so that a soft diet (1) a totally handsewn end-to-side anastomosis and (2) a totally
is begun on postoperative day 5 or 6. A barium swallow is per- stapled end-to-end anastomosis. The latter technique involves
formed on postoperative day 6 in preparation for hospital dis- opening the previously placed gastric staple line and advancing
charge on day 7 or 8. the handle of an end-to-end anastomosis (EEA) stapler through](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-17-320.jpg)
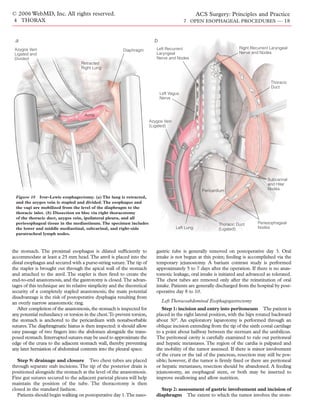
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 19
Stomach and
Esophagus
Approximated
with Single Stitch
Gastrostomy Made
Figure 19 Ivor-Lewis esophagectomy. The esophagus and the
with Electrocautery
proximal stomach are divided and stapled at least 5 cm away for Technique with
from the gross tumor. Stapled Anastomosis
Figure 20 Ivor-Lewis esophagectomy. The transposed stomach is
ach determines whether a total gastrectomy or a proximal gas- sutured to the adjacent pericardium, and a gastrotomy is carried
trectomy is indicated along with the distal esophagectomy. If no out halfway between the lesser curvature staple line and the
metastases are found, the incision is extended and the chest is greater curvature. The anastomosis is completed as in a transhi-
opened with a left posterolateral incision through the sixth inter- atal esophagectomy [see Figures 16 and 17].
space. If the thoracic component of the tumor appears to be
resectable, the costal margin is divided. It is advisable to remove a
Phrenic
1 to 2 cm segment of the costal margin to facilitate repair of the Nerve
diaphragm at the end of the operation and reduce postoperative
costal margin pain. The diaphragm is incised radially [see Figure
21]. Branches of the pericardiophrenic artery are suture-ligated,
and the sutures are left long so that they can be used as diaphrag-
matic retractors. Alternatively, a circumferential incision may be
made about 2 cm from the costal margin to reduce the risk of
postoperative diaphragmatic paralysis.
Step 3: division of pulmonary ligament and mobilization
of esophagus and stomach The pulmonary ligament is divid-
ed, and the mediastinal pleura is incised over the esophagus as far
as the aortic arch. The esophagus is mobilized above the tumor
and is retracted by a Penrose drain [see Figure 21].The esophageal Aorta
vessels are carefully dissected, ligated, and divided. The tumor is
mobilized; the plane of the dissection is kept close to the aorta on
the left, and if necessary, the right parietal pleura is taken in con-
tinuity with the lesion. About 1 cm of the crura is taken in conti- Diaphragm Sutures
nuity with the tumor to provide good local clearance. The stom-
ach is then mobilized in much the same way as in a transhiatal Figure 21 Left thoracoabdominal esophagogastrectomy. The
esophagectomy [see Figure 9]. diaphragm is incised radially. The branches of the pericardial
phrenic artery are suture-ligated, and the sutures are left long to
Step 4: assessment of pancreatic involvement and hepat- be used as diaphragmatic retractors. The pulmonary ligament is
divided, and the esophagus is mobilized above the tumor and
ic viability The lesser sac is opened through the greater omen-
retracted with a Penrose drain. The esophageal vessels are ligated
tum so that it can be determined whether the primary tumor and divided. The tumor and the esophagus are mobilized off the
involves the distal pancreas. If so, it is reasonable to resect the dis- aorta down to the hiatus; 1 cm of the diaphragmatic crura is
tal pancreas, the spleen, or both in continuity with the stomach; if taken in continuity with the tumor to provide local clearance. The
not, the short gastric vessels are ligated and divided, with the spleen stomach is then mobilized in much the same way as in a transhi-
preserved. The lesser omentum is detached from the right side of atal esophagectomy [see Figure 9].](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-19-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 20
Step 6: choice of partial or total gastrectomy At this
time, the surgeon determines whether the whole stomach must be
Aorta resected to remove the gastric part of the cancer or whether a par-
tial (i.e., proximal) gastrectomy will suffice.
Azygos
Vein
Proximal esophagogastrectomy with esophagogastrostomy. If the
surgeon decides that resection of the proximal stomach will re-
move all of the tumor while leaving at least 5 cm of tumor-free
stomach, a proximal esophagogastrectomy is performed [see Figure
22]. A gastric tube is fashioned with a linear stapler [seeTranshiatal
Esophagectomy, Step 9, above, and Figure 15]. The staple line is
Inferior oversewn with inverting 3-0 sutures. Because the vagus nerves are
Pulmonary
Vein
divided and gastric stasis may result, a pyloromyotomy is per-
formed, much as in a transhiatal esophagectomy.
The proximal gastric resection margin is covered with a sponge
and turned upward over the costal margin. The stomach tube is
then brought up through the hiatus and into the thorax behind the
proximal esophageal resection margin. The margin should be at
least 10 cm from the proximal end of the esophagogastric cancer.
If the esophageal resection margin is not adequate, the stomach
tube is mobilized and brought to the left neck, then anastomosed
to the cervical esophagus through a left neck incision; alternative-
ly, the left colon is interposed between the gastric stump and the
cervical esophagus. If the resection margin is adequate, the tip of
the stomach tube is sewn to the posterior wall of the esophagus
[see Figure 23].
Figure 22 Left thoracoabdominal esophagogastrec-
tomy: proximal esophagogastrectomy with esopha-
gogastrostomy. If the tumor can be completely
resected by removing the proximal stomach, a prox-
imal esophagogastrectomy is carried out.
the esophagus and the hilum of the liver, then divided down to the
area of the pylorus, with the right gastric artery and vein preserved.
There is often a hepatic branch from the left gastric artery running
through the gastrohepatic omentum. If this hepatic branch is of sig-
nificant size, a soft vascular clamp should be placed on the artery
for 20 minutes so that the viability of the liver can be assessed. If
the liver is viable, the artery is suture-ligated and divided.
Step 5: division of greater omentum and short gastric
vessels The greater omentum is divided, with care taken to pre-
serve the right gastroepiploic artery and vein.These two vessels are
suture-ligated and divided well away from the stomach. Ligation
and division of the short gastric vessels allow complete mobiliza-
tion of the greater curvature of the stomach. Dissection is extend- Figure 23 Left thoracoabdominal esophagogastrectomy: proxi-
ed downward as far as the pylorus.The stomach is turned upward, mal esophagogastrectomy with esophagogastrostomy. The esoph-
and the left gastric vessels are exposed through the lesser sac [see agus is sewn to the tip of the stomach tube, halfway between the
Figure 10].The lymph nodes along the celiac axis and the left gas- lesser curvature suture line and the greater curvature. An anasto-
tric artery are swept up into the specimen, and the gastric vessels mosis is then fashioned with the stapling technique used in trans-
are either suture-ligated or stapled and divided. hiatal esophagectomy [see Figures 16 and 17].](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-20-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 21
The anastomosis is then performed with the stapling technique
previously described for transhiatal esophagectomy [see Figures 16
and 17]. A nasogastric tube is passed down into the gastric rem-
nant. The tube is sewn to the pericardium and the endothoracic
fascia to prevent anastomotic dehiscence.
Total gastrectomy with Roux-en-Y esophagojejunostomy. If the
surgeon decides that a total gastrectomy is necessary, the right gas-
troepiploic and right gastric vessels are suture-ligated and divided
distal to the pylorus. The duodenum is divided just distal to the
pylorus with a linear stapler.The staple line is inverted with inter-
rupted 3-0 nonabsorbable sutures and covered with omentum to
prevent duodenal stump blowout.
The esophagus is then mobilized up to the level of the inferior
pulmonary vein. Two retaining sutures are placed in the esoph-
ageal wall. A monofilament nylon purse-string suture is placed
around the circumference of the proximal esophagus in prepara-
tion for stapling. A No. 24 Foley catheter with a 20 ml balloon is
advanced into the esophagus and gently inflated to distend the
esophageal lumen. The resected specimen is sent to the patholo-
gist for examination of the margins.
A jejunal interposition is then fashioned by using the Roux-en-
Y technique. One or two jejunal arteriovenous arcades are divided
to mobilize enough jejunum to allow anastomosis to the thoracic
esophagus [see Figure 24]. After removal of the Foley catheter, a 25
or 28 mm EEA stapler is passed through the jejunum into the
esophagus, fired, and removed. The jejunum is anchored to the
pericardium and the proximal esophagus. The duodenal loop is
anastomosed to the jejunum at least 45 to 50 cm distal to the
esophagojejunal anastomosis to minimize bile reflux [see Figure
24].The blind end of the jejunal loop is then stapled closed.
After careful irrigation of the chest, the first step in the closure is
to repair the diaphragm around the hiatus. The gastric or jejunal
interposition is sewn to the crura with interrupted nonabsorbable Figure 24 Left thoracoabdominal esophagogastrectomy: total
sutures. The remainder of the diaphragm is closed with interrupt- gastrectomy with Roux-en-Y esophagojejunostomy. A jejunal
ed nonabsorbable 0 mattress sutures. A chest tube is placed into interposition is fashioned with the Roux-en-Y technique. One or
the pleural space close to but not touching the anastomosis. The two jejunal arteriovenous arcades are divided to mobilize enough
final sutures in the peripheral part of the diaphragm are placed but jejunum for anastomosis to the thoracic esophagus. A 25 or 28
are not tied until the ribs are brought together with pericostal mm EEA stapler is passed through the jejunum into the esopha-
gus. The jejunum is anchored to the pericardium and the proxi-
sutures. The left lung is reexpanded. The costal cartilages are not
mal esophagus. The duodenal loop is anastomosed to the jejunum
approximated but are left to float free. If the ends of the costal mar- at least 45 to 50 cm distal to the esophagojejunal anastomosis. The
gin are abutting, another 2 cm of costal cartilage should be blind end of the jejunal loop is then stapled closed.
removed to reduce postoperative pain. Thoracic and abdominal
skin layers are closed with a continuous absorbable suture. The
skin and the subcutaneous tissue are closed in the usual fashion. to minimize postprandial dumping. Patients are also taught how
to care for their temporary feeding jejunostomy. Consumption of
POSTOPERATIVE CARE caffeine and carbonated beverages is usually limited during the
As a rule, patients are not routinely admitted to the ICU after first few weeks after discharge.
esophagectomy; however, individual practices depend on the dis- A barium swallow is performed on postoperative day 7 to veri-
tribution of skilled nursing and physiotherapy personnel. Early fy the integrity of the anastomosis and the patency of the
ambulation is the mainstay of postoperative care. As a rule, pyloromyotomy. Patients are usually discharged on postoperative
patients are able to walk slowly, with assistance, on postoperative day 7 or 8. The feeding jejunostomy is left in place until the first
day 1. Patient-controlled epidural analgesia is particularly useful postoperative evaluation, which usually takes place 2 to 3 weeks
in facilitating good pulmonary toilet and minimizing the risk of after the operation. The feeding tube is removed during that visit
atelectasis or pneumonia. if oral intake and weight are stable.
The nasogastric tube is removed on postoperative day 3;
COMPLICATIONS OF ESOPHAGECTOMY
jejunostomy tube feedings are gradually started at the same time.
Once bowel function normalizes, patients are allowed small sips of
liquids. Chest tubes are removed as pleural drainage subsides. By Pulmonary Impairment
postoperative day 6, most patients have progressed to a soft solid Atelectasis and pneumonia should be considered preventable
diet. Dietary education is provided, focusing primarily on eating complications of esophagectomy. Patients with recognized preop-
smaller and more frequent meals, avoiding bulky foods (e.g., meat erative impairment of pulmonary reserve should be considered for
and bread) in the early postoperative period, and taking measures transhiatal esophagectomy. Existing pulmonary function can be](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-21-320.jpg)

![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 23
tion and cause. The cervical anastomotic leaks that may develop problems and are difficult to manage; those presenting later are
after transhiatal esophagectomy are generally simple to treat. generally related to some degree of ischemic tissue loss. Patients
Leaks in the early postoperative period are usually related to tech- who have received radiation therapy or are nutritionally depleted
nical factors (e.g., excessive tension across the anastomosis). A may be especially vulnerable to problems with anastomotic heal-
nonviable stomach may not give rise to obvious signs, and thus, ing. A contrast swallow with dilute barium is the best method of
any possibility of ischemia in the transposed stomach must be evaluating the anastomosis. Leaks may be manifested either as a
addressed promptly.Tachycardia, confusion, leukocytosis, cervical free flow of contrast into the pleural space or as a contained fluid
wound drainage, and neck tenderness may or may not be present. collection.
The morbidity of an open cervical wound is not high—cer- Small leaks that drain immediately into properly placed thora-
tainly lower than that of an untreated leak. Accordingly, any clin- costomy tubes can usually be managed by giving antibiotics and
ical suspicion of a leak should prompt a diagnostic contrast swal- withholding oral intake. Local control of infection generally results
low study using dilute barium. Large leaks are manifested as in spontaneous healing. Anastomotic disruptions that are large or
persistent collections of contrast material outside the esophagus. are associated with a major pleural collection typically necessitate
Although such leaks rarely extend into the pleural space, any open drainage with decortication; percutaneous drainage may be
fluid in the chest must be drained so that its nature can be deter- considered as a preliminary approach in selected patients.
mined. The neck wound is opened by removing the sutures and Persistent soiling of the mediastinum and the pleural space is fatal
performing gentle digital exploration of the prevertebral space if untreated.
behind the esophagus as the finger is advanced into the medi- Early esophagoscopy is strongly advised to evaluate the viabili-
astinum; this is usually done at the bedside and requires little, if ty of the gastric remnant. Ischemic necrosis of the stomach neces-
any, patient sedation. sitates reexploration, decortication, takedown of the anastomosis,
Saline-moistened gauze packing is changed three or four times gastric debridement, return of any viable stomach into the abdo-
a day. Prolonged or copious cervical drainage may call for supple- men, closure of the hiatus, and proximal diversion with a cervical
mental deep wound aspiration with a Yankauer suction handle. esophagostomy. Repair or revision of the anastomosis in an infect-
Administering water orally during aspiration facilitates removal of ed field is certain to fail and should never be considered. In cer-
any necrotic debris. A fetid, malodorous breath associated with tain cases, diversion via a cervical esophagostomy and a comple-
sanguineous discharge from the nasogastric tube and purulent tion gastrectomy may be required.
fluid in the opened neck incision are ominous signs that should
prompt early esophagoscopy. Diffuse mucosal ischemia may indi- Late Complications
cate the presence of a nonviable stomach; reoperation with com- At every postoperative visit, symptoms of reflux, regurgitation,
pletion gastrectomy and proximal esophagostomy is required to dumping, poor gastric emptying, and dysphagia must be specifi-
treat this rare catastrophic complication. cally sought: these are the major quality-of-life issues for post-
Generally, leaks that occur more than 7 days after operation are esophagectomy patients.4 Reflux and regurgitation may complicate
small and are related to some degree of late ischemic disruption any form of alimentary reconstruction after esophagectomy, though
along the anastomosis. They can usually be managed by opening cervical anastomoses are less likely to be associated with sympto-
the cervical wound at the bedside and packing the site with gauze. matic reflux than intrathoracic anastomoses are. Reflux symptoms
Oral diet is advanced as tolerated. It may be noted that the volume generally respond to dietary modifications, such as smaller and
of the leak is markedly greater or less depending on the position of more frequent meals. Regurgitation is usually related to the supine
the head during swallowing. Accordingly, before discharge, patients position and thus tends to be worse at night; elevating the bed and
are taught how to temporarily adjust their swallowing as well as avoiding late meals may suffice for symptom control. Dumping is
how to manage their dressing changes. Applying gentle pressure to exacerbated by foods with high fat or sugar content. Dysphagia
the neck wound and turning the head to the left may help the may be related to narrowing at the anastomosis or, in rare instances,
patient ingest liquids with minimal soiling of the open neck inci- to poor emptying of the transposed stomach.Anastomotic strictures
sion. Dysphagia, even with an opened neck incision, should be are most commonly encountered as a sequel to a postoperative
treated by passing tapered esophageal dilators orally between 2 and leak.There may be excessive scarring at the anastomosis, associat-
4 weeks after surgery.When a bougie at least 48 French in caliber ed with local distortion or angulation. Specific tests for gastric atony
can be passed through the anastomosis, the patient can usually include nuclear medicine gastric emptying studies using radiola-
swallow comfortably.The size of the leak often decreases after dila- beled food. A simple barium swallow may indicate an incomplete
tion as food is allowed to proceed preferentially into the stomach. pyloromyotomy as a cause of poor gastric emptying; balloon dila-
To maximize the diameter of the anastomosis and reduce the like- tion often corrects this problem.
lihood of a symptomatic stricture, subsequent dilations should be Any form of anastomotic leak will increase the incidence of late
scheduled at 2-week intervals for the next few months. stricture. Dysphagia may be treated by means of progressive dila-
When a routine predischarge barium swallow after transhiatal tion with Maloney bougies. This procedure is performed in the
esophagectomy raises the possibility of an anastomotic leak in an outpatient clinic and often does not require sedation or any other
asymptomatic patient, the question arises of whether the wound special patient preparation. Complications are rare if due care is
should be opened at all. For small, contained leaks associated with exercised during the procedure. As noted [seeTransthoracic Hiatal
preferential flow of contrast material into the stomach, observa- Hernia Repair, Preoperative Evaluation, Dilation, above], it is
tion alone may suffice in selected cases. Patients must be closely essential that the caliber of the dilators be increased gradually and
watched for fever or other signs of major infection. Given the quite that little or no force be applied in advancing them. The appear-
low morbidity of cervical wound exploration, the surgeon should ance of blood on a withdrawn dilator signals a breach of the
not hesitate to drain the neck if the patient’s condition changes. mucosa; further dilation should be done cautiously lest a trans-
The incidence of anastomotic leakage is low after Ivor-Lewis mural injury result. Comfortable swallowing, of liquids at least, is
resection, but the consequences are significant. Leaks presenting usually achieved after the successful passage of a 48 French
early in the postoperative period are usually related to technical bougie. It is preferable, however, to advance dilation until at least](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-23-320.jpg)
![© 2006 WebMD, Inc. All rights reserved. ACS Surgery: Principles and Practice
4 THORAX 7 OPEN ESOPHAGEAL PROCEDURES — 24
a 54 French bougie can be passed with ease. For late strictures that with a totally handsewn anastomosis. As regards postoperative
are particularly difficult to dilate, endoscopic examination and his- function, stomach interposition through the posterior medi-
tologic evaluation may be required to rule out a recurrent tumor. astinum after transhiatal esophagectomy is associated with low
CT of the chest should also be performed whenever there is unex- rates of aspiration and regurgitation. Esophageal reflux and
plained weight loss or fatigue late after esophagectomy. esophagitis—commonly seen with intrathoracic esophagogastric
The Savary system of wire-guided dilators has been particular- anastomoses—are usually not clinically significant problems with
ly helpful in the management of tight or eccentric strictures. this approach. Patients are advised to elevate the head of their bed
Patients are generally treated in the endoscopy suite. Temporary and to continue taking PPIs for about 3 months after the opera-
sedation with I.V. fentanyl and midazolam is required. Fluoroscopy tion. Approximately one third will require esophageal dilation for
is used to confirm proper placement of a flexible-tip wire across dysphagia after the operation. Some 7% to 10% experience post-
the stricture. Serial wire-guided dilation can then be performed vagotomy dumping symptoms, which in most cases can be con-
with confidence and increased patient safety. trolled by simply avoiding high-carbohydrate foods and dairy
products.
OUTCOME EVALUATION
Ivor-Lewis Esophagectomy
Transhiatal Esophagectomy Ivor-Lewis esophagectomy is associated with anastomotic leak-
A 1999 study from the University of Michigan presented data age rates and operative mortalities of less than 3%.3,6 Approxi-
on 1,085 patients who underwent transhiatal esophagectomy mately 5% of patients will require anastomotic dilation. Again,
without thoracotomy, of whom 74% had carcinoma and 26% had patients are advised to elevate the head of the bed and to contin-
nonmalignant disease.5 Transhiatal esophagectomy was completed ue taking PPIs. In some patients, the gastric interposition rotates
in 98.6% of the patients; the remaining 1.4% were converted to a into the right posterolateral thoracic gutter, resulting in postpran-
transthoracic esophagectomy as a result of either thoracic esoph- dial gastric tension and rendering them more susceptible to aspi-
ageal fixation or bleeding. Previous chemotherapy or radiation ration. Compared with transhiatal esophagectomy, extended
therapy did not preclude performance of a transhiatal esophagec- transthoracic esophagectomy is associated with higher pulmonary
tomy. Nine patients experienced inordinate intraoperative blood morbidity and operative mortality but also with a superior 3-year
loss; three died as a result. The overall hospital mortality was 4%. survival rate.7
The overall 5-year survival rate for patients undergoing transhiatal
esophagectomy is approximately 20% for adenocarcinoma of the Left Thoracoabdominal Esophagogastrectomy
cardia and the esophagus and 30% for squamous cell carcinoma Left thoracoabdominal esophagogastrectomy is also associated
of the esophagus. with anastomotic leakage rates and operative mortalities of less
The stapled anastomosis described earlier [see Operative than 3%.8 Approximately 5% of patients will require esophageal
Technique, Transhiatal Esophagectomy, Step 8, above] reflects dilation. Reconstructions involving anastomosis of the distal stom-
numerous refinements introduced at the University of Michigan. ach to the esophagus are associated with a high incidence of
The endoscopic GIA stapler has a low-profile head that is ideally delayed gastric emptying and bile gastritis and esophagitis. Of all
suited to the tight confines of the neck, enabling the surgeon to the operations we have described, this one results in the lowest
fashion a widely patent side-to-side functional end-to-end anasto- postoperative quality of life. Accordingly, most surgeons prefer to
mosis with three rows of staples along the back wall. The rate of carry out a total gastrectomy. Swallowing is restored with a Roux-
anastomotic stricture is markedly lower with this anastomosis than en-Y jejunal interposition.
References
1. Lahey FH, Warren K: Esophageal diverticula. Surg nal function following esophagectomy for malig- transthoracic resection compared with limited tran-
Gynecol Obstet 98:1, 1954 nancy. Am J Surg 169:471, 1995 shiatal resection for adenocarcinoma of the esoph-
agus. N Engl J Med 347:1662,2002
2. Stirling MC, Orringer MB: Continued assessment 5. Orringer MB, Marshall B, Iannettoni MD:
of the combined Collis-Nissen operation. Ann Transhiatal esophagectomy: clinical experience and 8. Akiyama H, Miyazono H, Tsurumaru M, et al:
Thorac Surg 47:224, 1989 refinements. Ann Surg 230:392, 1999 Thoracoabdominal approach for carcinoma of the
cardia of the stomach. Am J Surg 137:345, 1979
3. Mathiesen DJ, Grillo HC, Wilkens EW Jr: 6. King RM, Pairolero PC, Trastek VF, et al: Ivor
Transthoracic esophagectomy: a safe approach to Lewis esophagogastrectomy for carcinoma of the
carcinoma of the esophagus. Ann Thorac Surg esophagus: early and long-term results. Ann Thorac Acknowledgment
45:137, 1988 Surg 44:119, 1987
4. Finley RJ, Lamy A, Clifton J, et al: Gastro-intesti- 7. Hulscher JBF, van Sandick JW et al: Extended Figures 1 through 24 Tom Moore.](https://image.slidesharecdn.com/acs0407-openesophagealprocedures-100726063211-phpapp02/85/Acs0407-Open-Esophageal-Procedures-24-320.jpg)