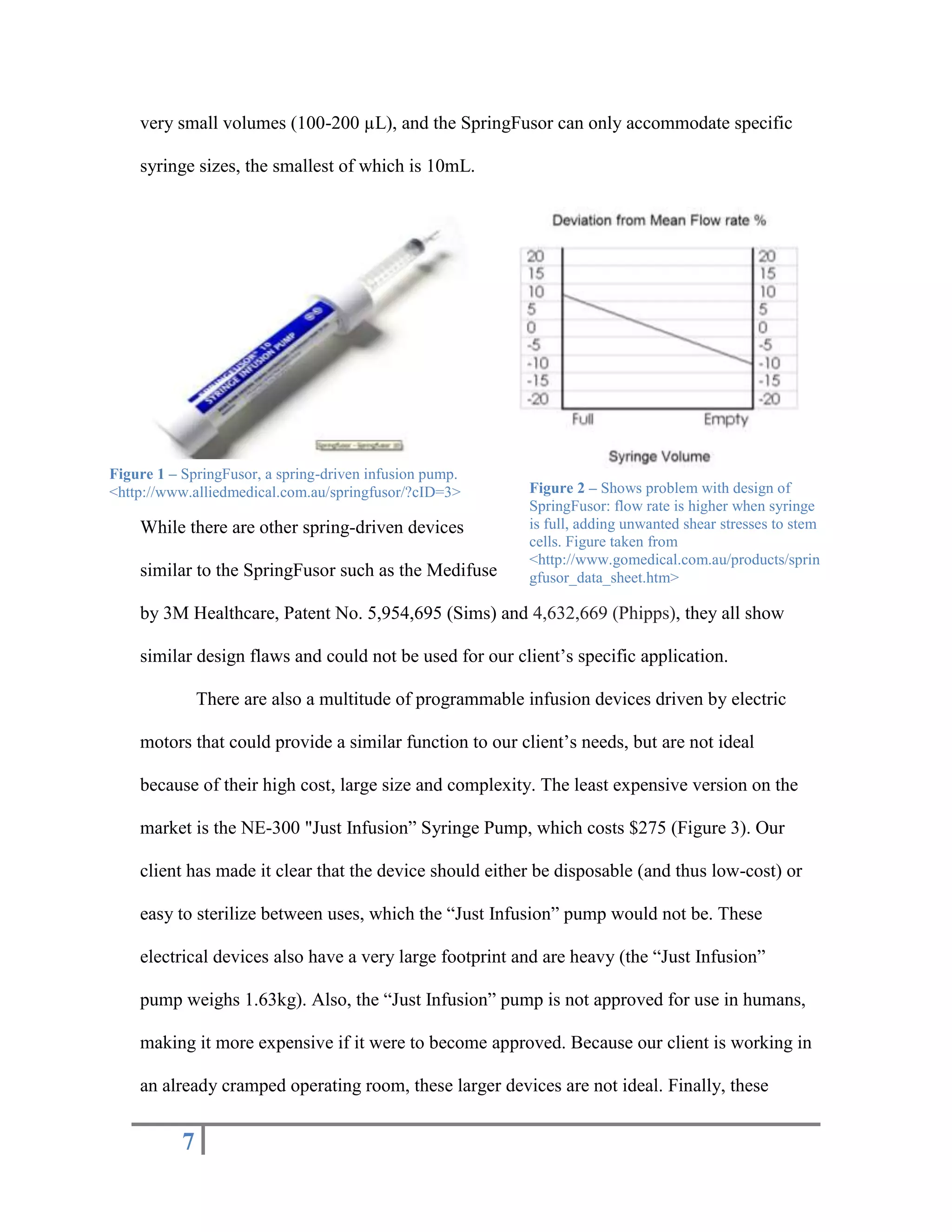
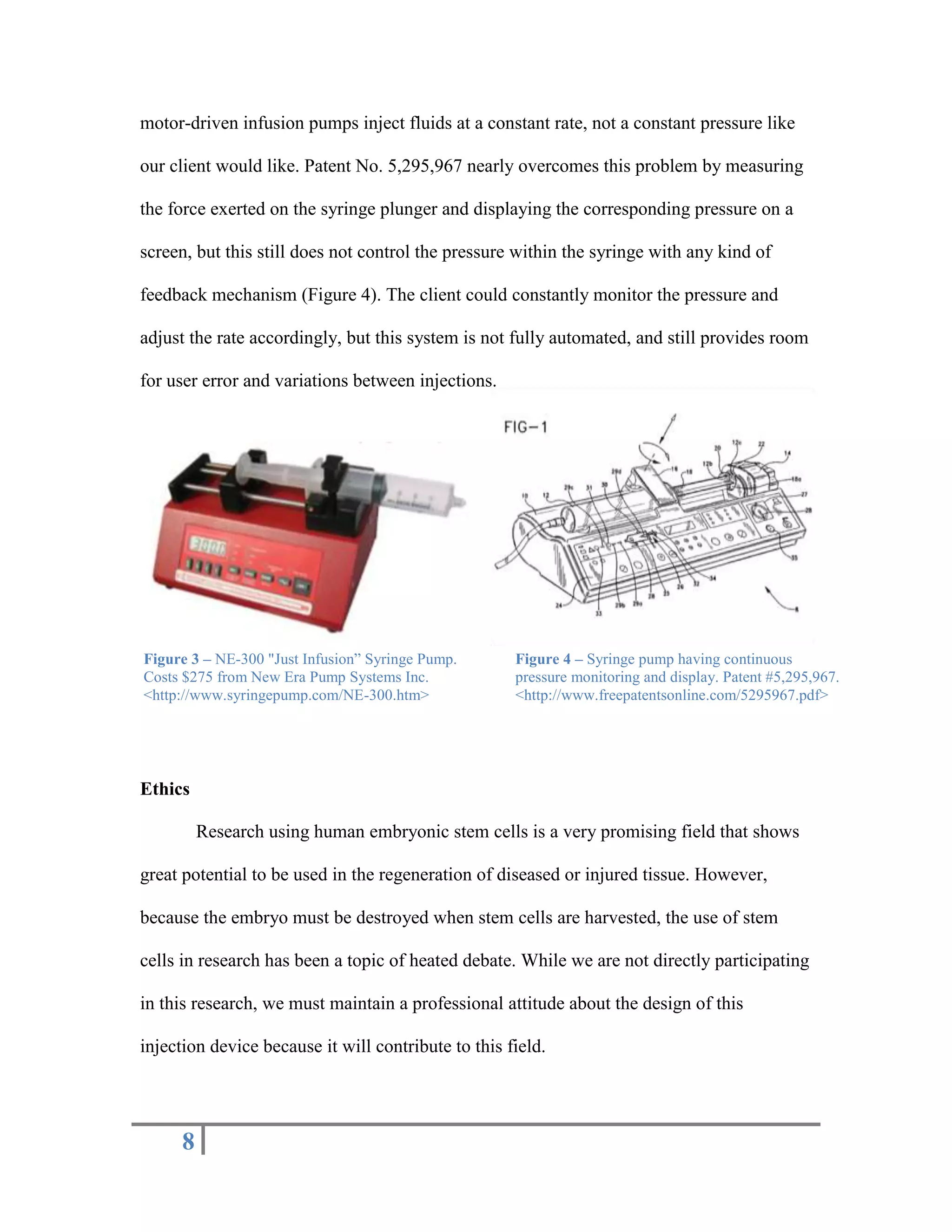
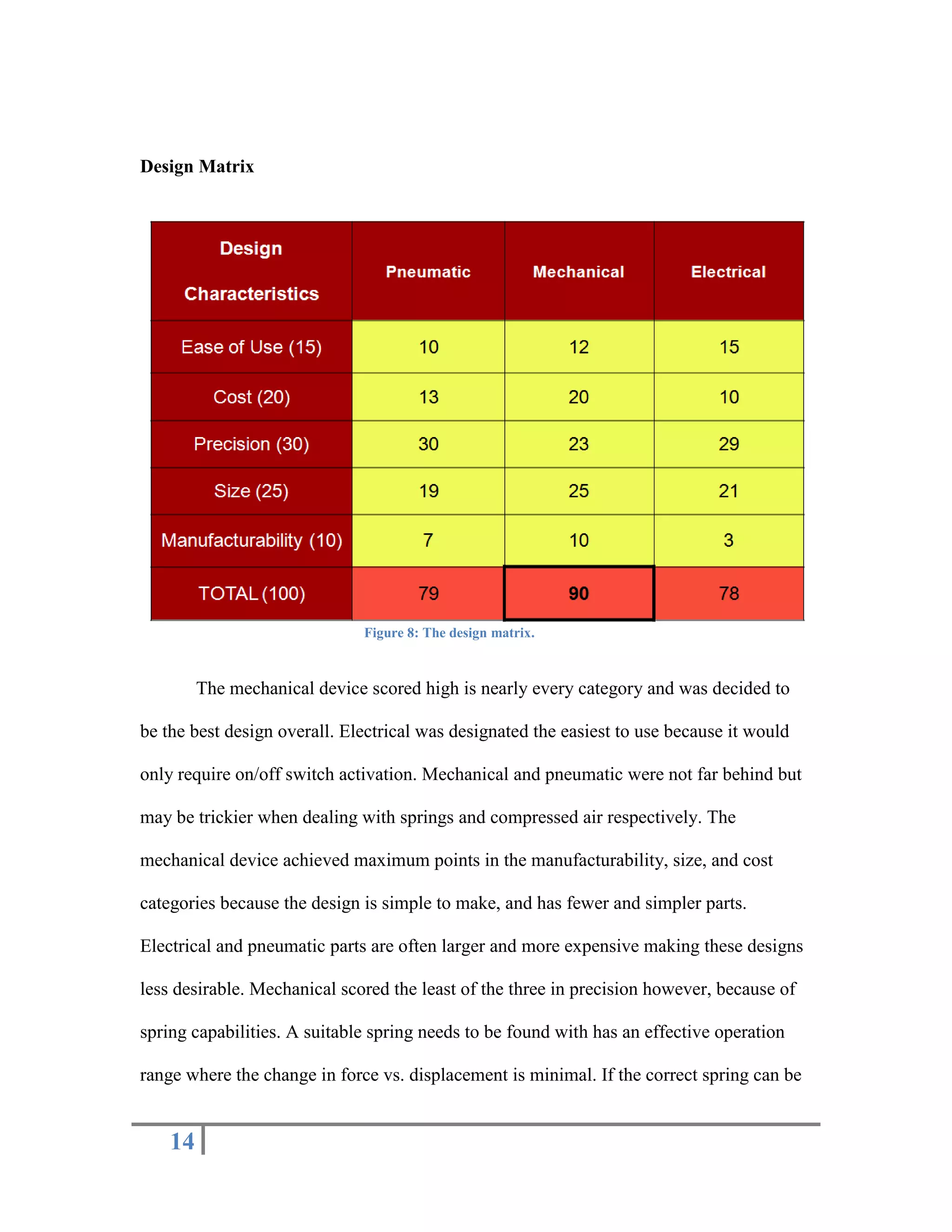
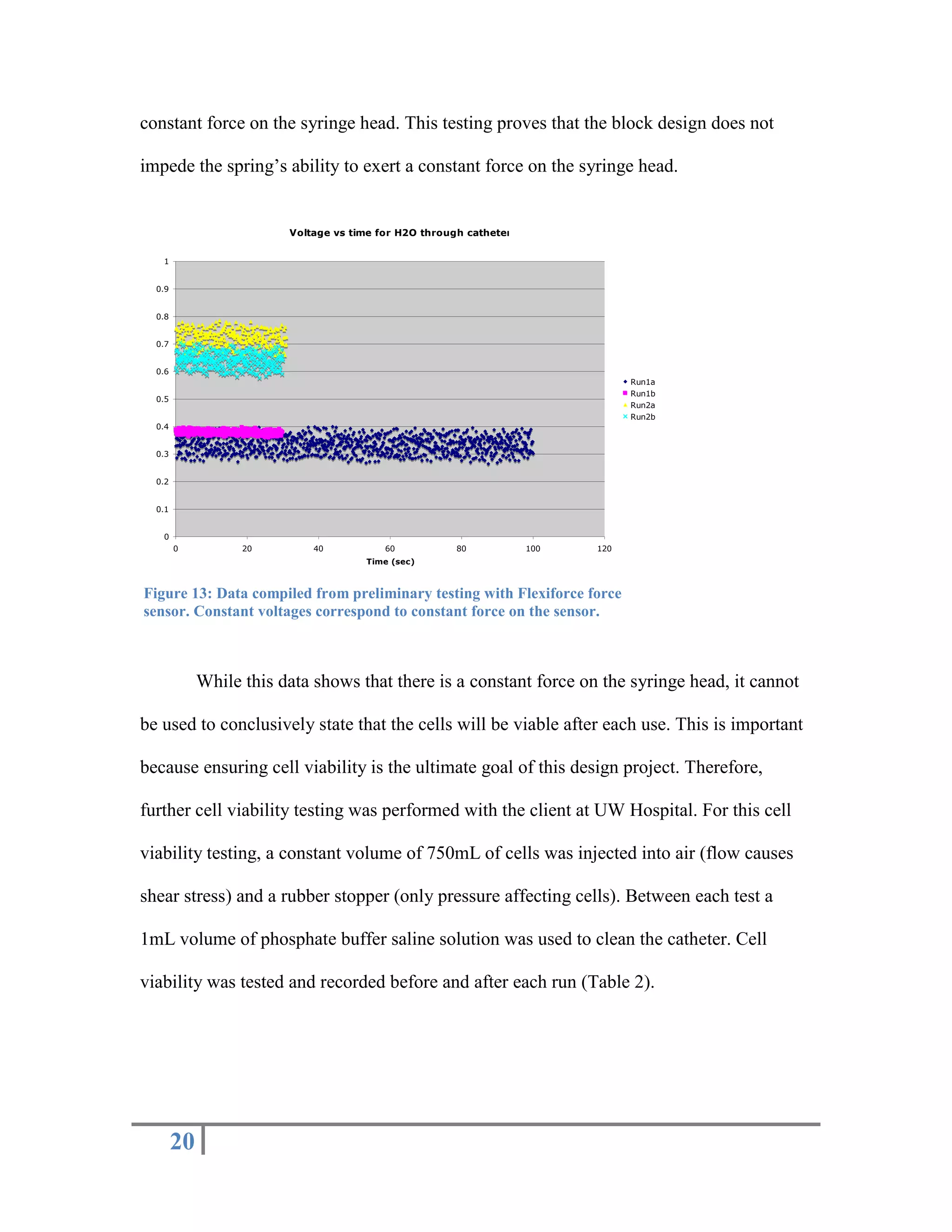
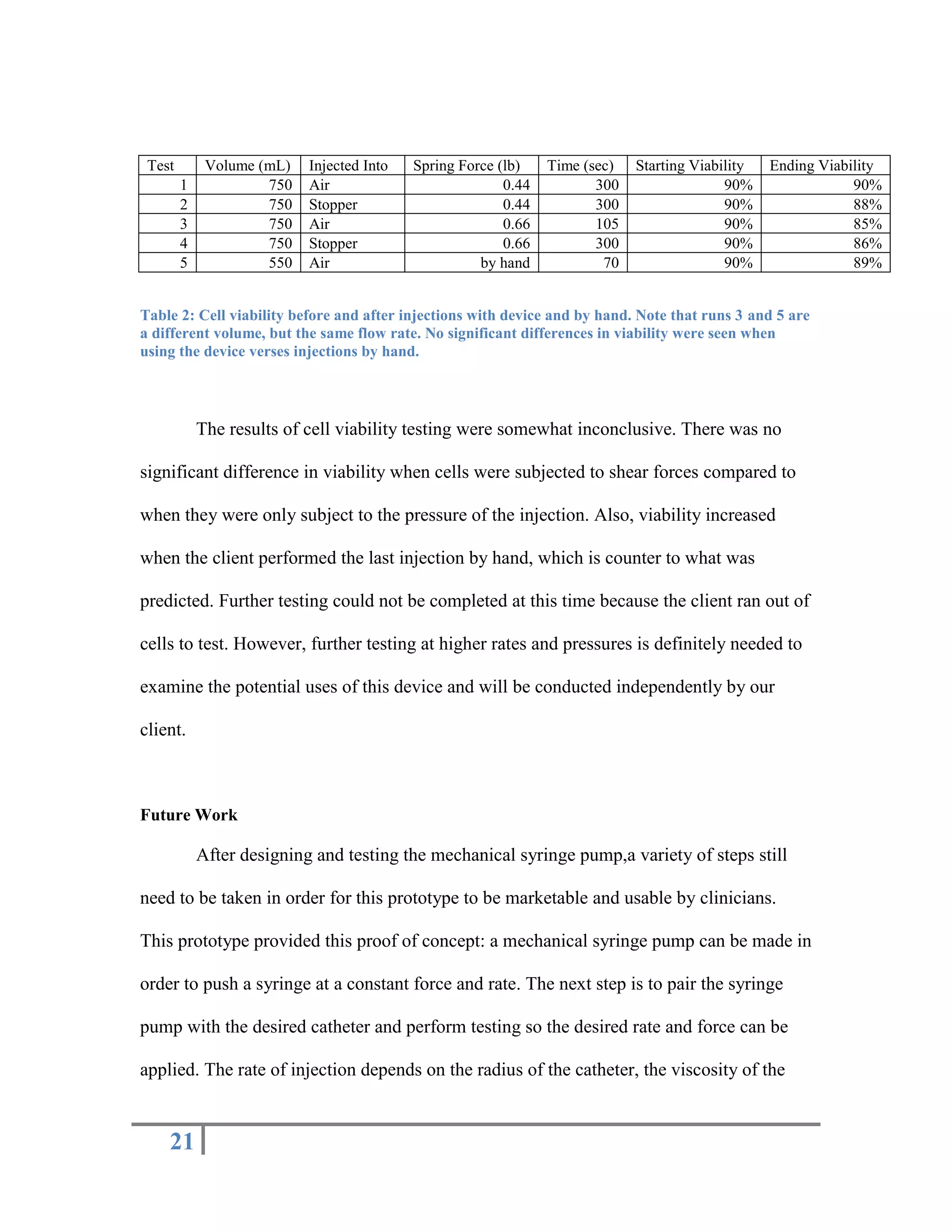
The document outlines the design of a precise handheld injection device aimed at improving the accuracy of stem cell injections for cardiac interventions. The current manual method leads to variability in pressure, risking cell viability, hence the need for an automated device that maintains constant pressure. Three design proposals—pneumatic, electrical, and mechanical—were evaluated, with the mechanical design emerging as the most favorable due to its simplicity, cost-effectiveness, and suitability for clinical use.