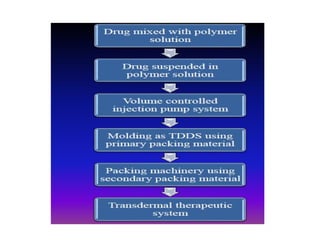
There are four main types of transdermal drug delivery systems (TDDS):
1. Polymer membrane permeation-controlled
2. Polymer matrix diffusion-controlled
3. Drug reservoir gradient-controlled
4. Micro reservoir dissolution-controlled
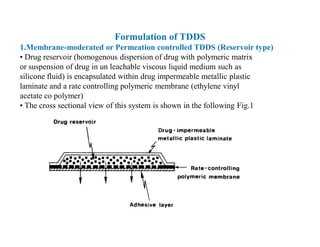
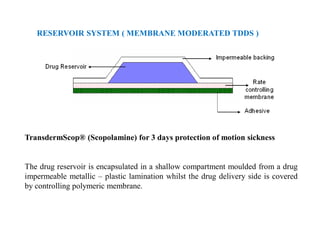
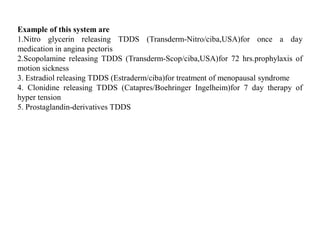
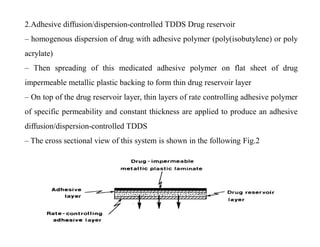
The main formulations of TDDS include membrane-moderated or permeation controlled systems and adhesive diffusion/dispersion-controlled systems. Membrane-moderated systems use a drug reservoir encapsulated by an impermeable backing and a rate-controlling membrane, while adhesive diffusion-controlled systems directly disperse the drug in an adhesive polymer layer applied to an impermeable backing. Examples provided are transdermal patches for conditions like angina, hypertension,