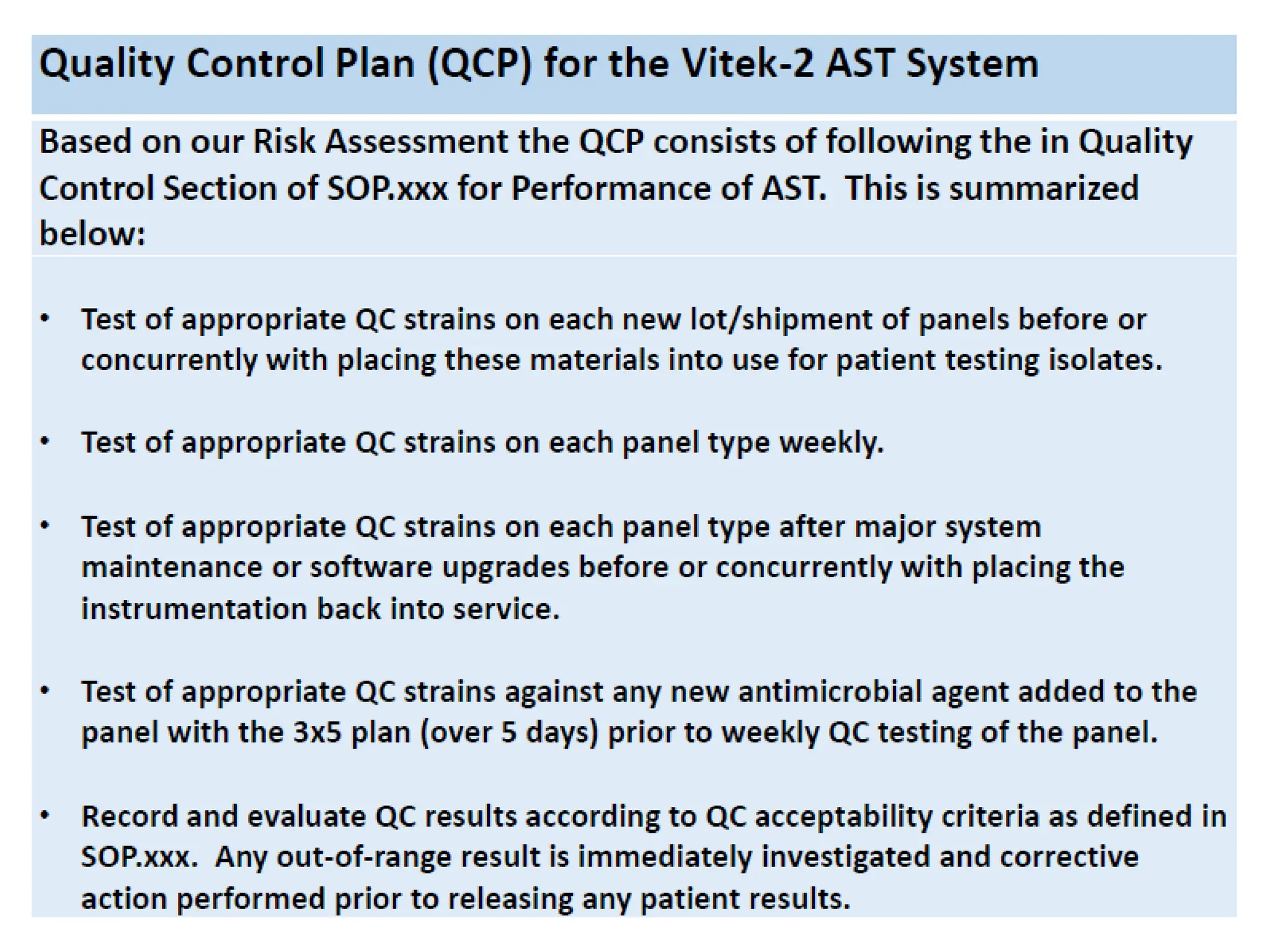
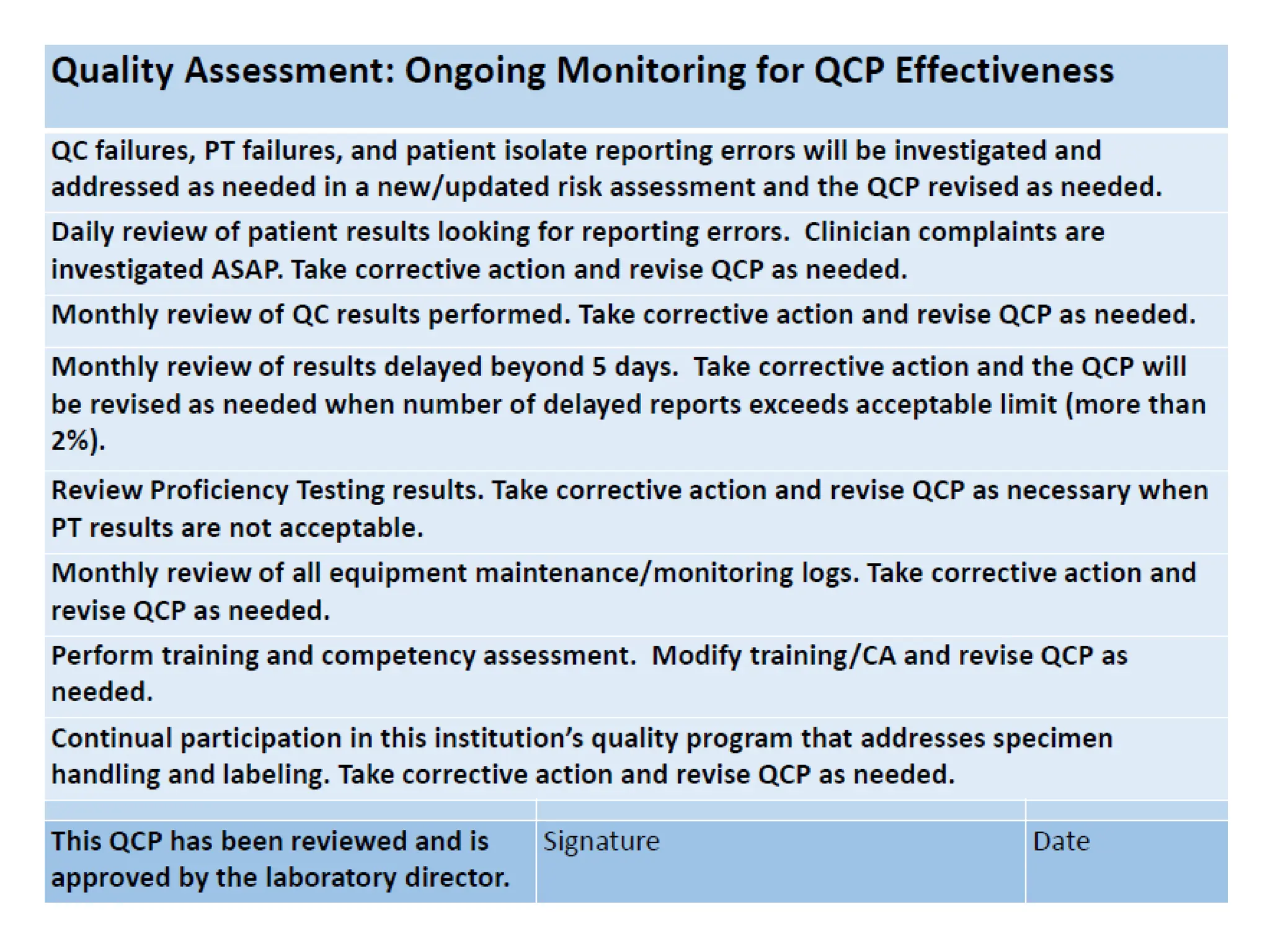
The document explains the Individualized Quality Control Plan (IQCP), its objectives, components, and benefits for laboratories. It details the processes to create an IQCP, including risk assessment and the use of computer programs for guidance. Implementing IQCP allows for customized quality control but poses risks if not managed properly.