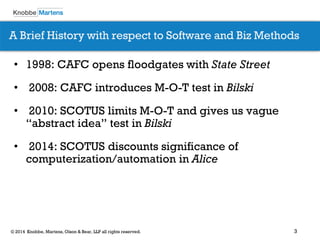
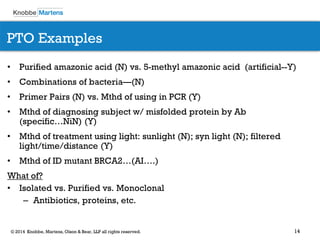
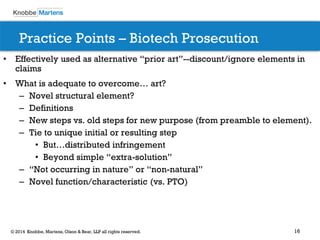
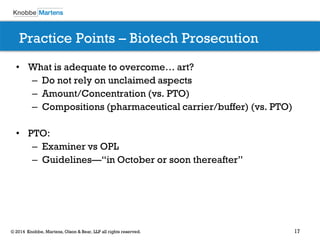
The document covers recent developments in patent eligibility for software, IT, and biotechnology, particularly following key Supreme Court rulings. It discusses the challenges and strategies for responding to patent rejections under the abstract idea test and the Mayo framework, emphasizing the importance of drafting claims that highlight unique features beyond abstract concepts. Additionally, it outlines practice points for navigating the complexities of patent prosecution in light of evolving standards and interpretations.