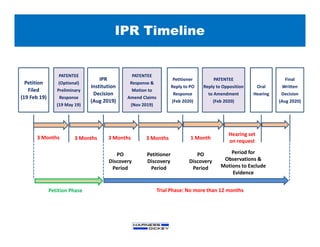
The document provides information on intellectual property (IP) practice, including:
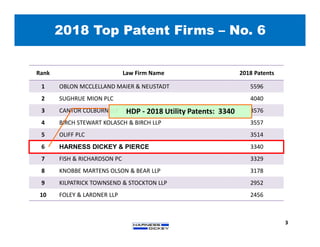
1) HDP is the 10th largest US IP firm with over 110 attorneys across four offices. In 2018 it obtained the 6th highest number of utility patents.
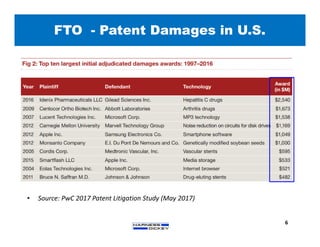
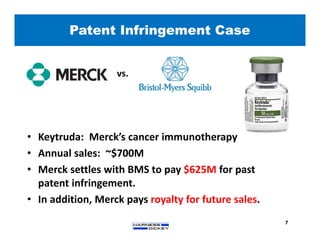
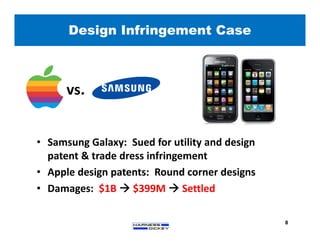
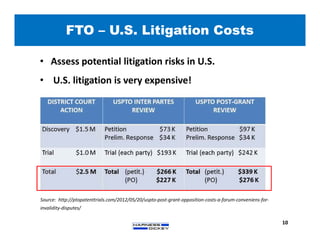
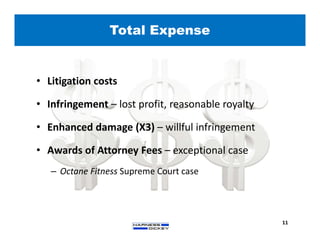
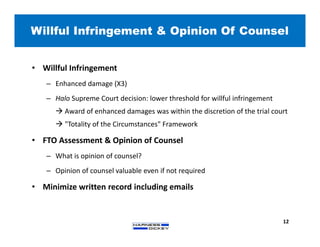
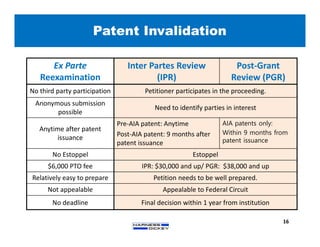
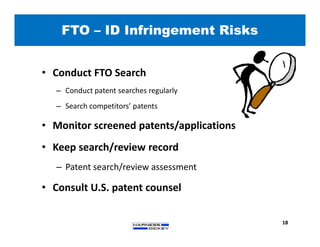
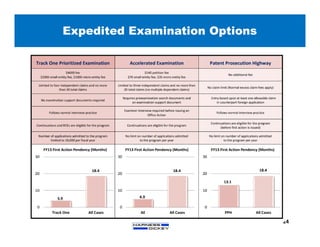
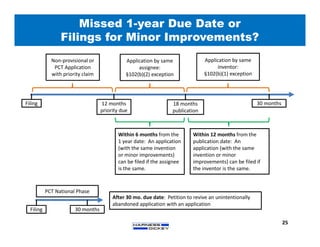
2) It discusses strategies for freedom to operate (FTO) analyses such as patent searches, monitoring risks of infringement, and obtaining opinions of counsel. Litigation costs and risks are also reviewed.
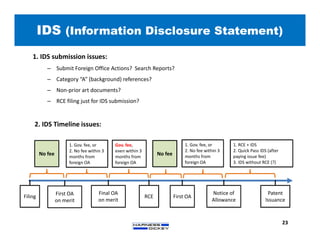
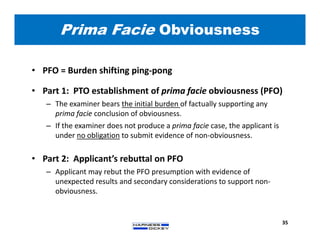
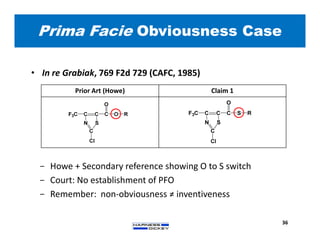
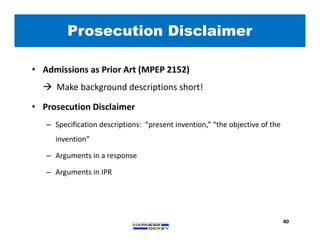
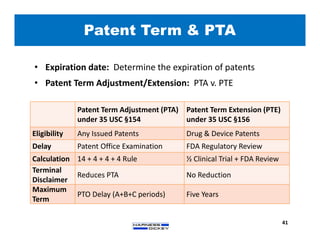
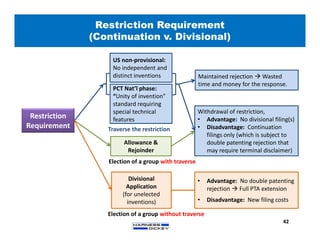
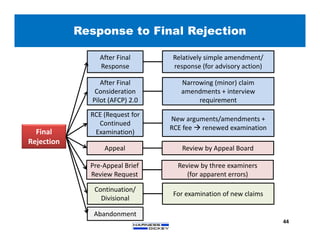
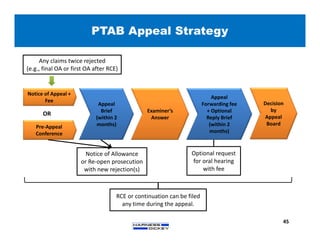
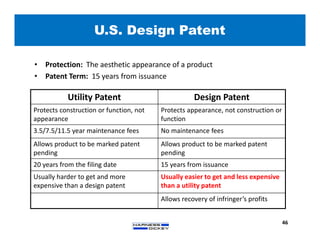
3) Guidelines are presented for patent preparation and prosecution best practices, including ownership issues, duty of candor, claim drafting, means-plus-function language, and obviousness arguments.