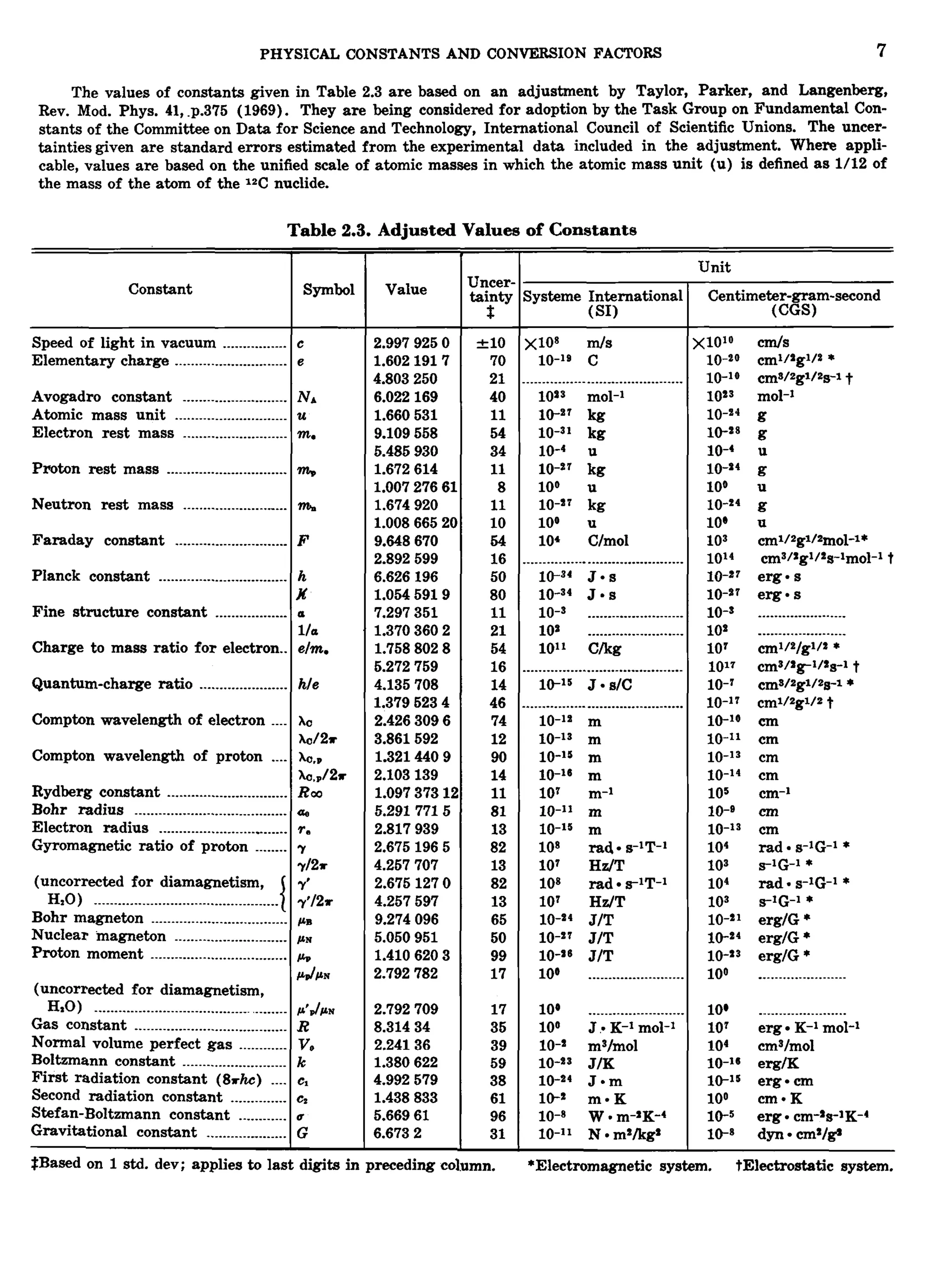
This document contains tables of physical constants, conversion factors, and other reference information. Table 2.1 lists common units in the SI and CGS systems and conversion factors between them. Table 2.2 provides names and conversion factors for electric and magnetic units in SI, emu, and esu systems. Table 2.3 gives adjusted values of fundamental physical constants with uncertainties. Tables 2.4-2.6 provide additional conversion factors, constants, and geodetic information.