This document covers key concepts in scientific measurement including:
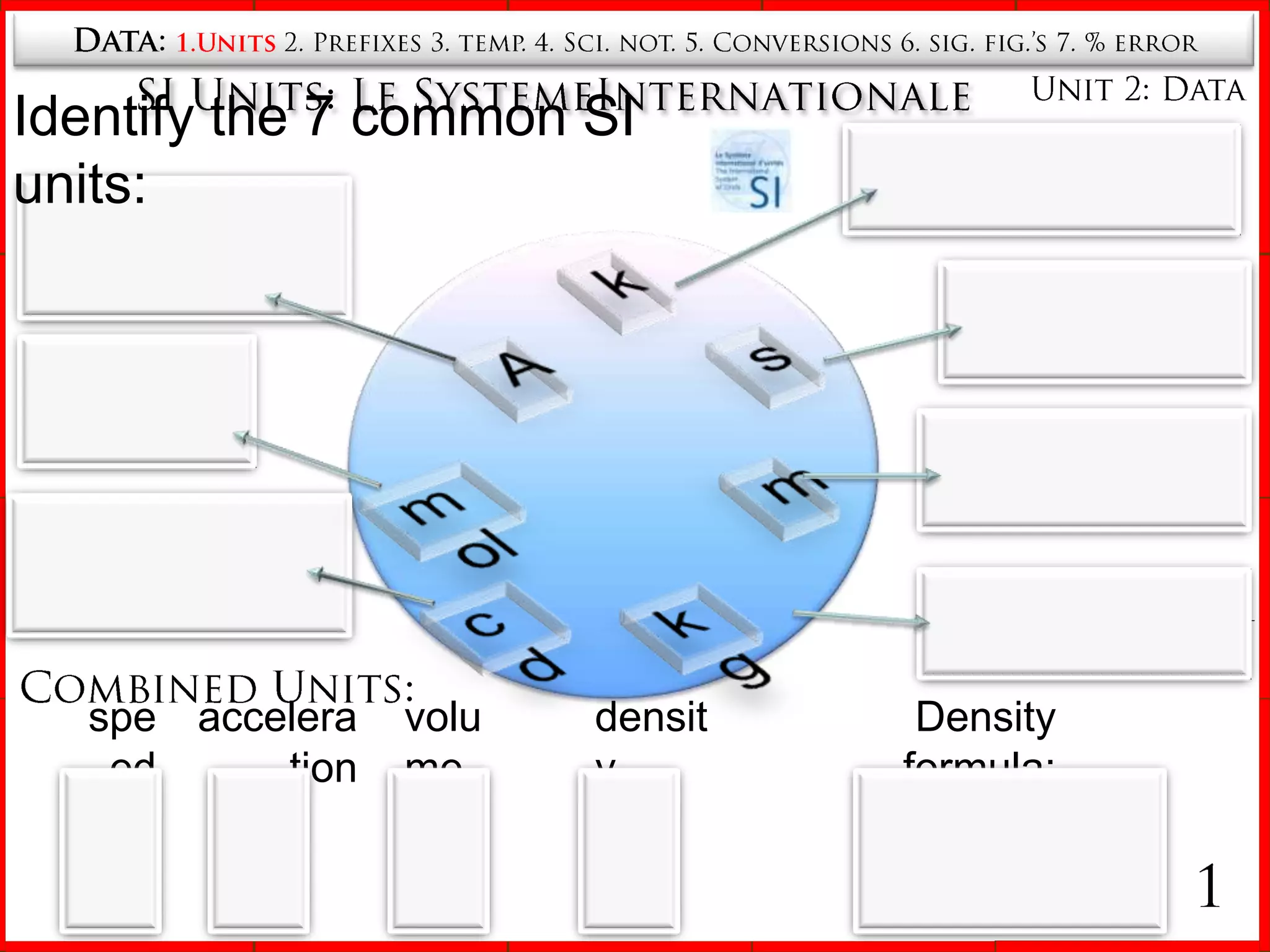
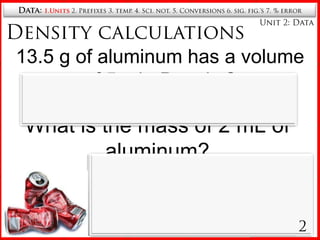
1. The seven SI base units including meters, kilograms, seconds, etc.
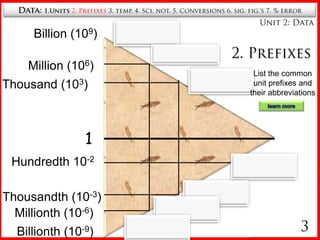
2. Common unit prefixes like milli, centi, and kilo and their abbreviations.
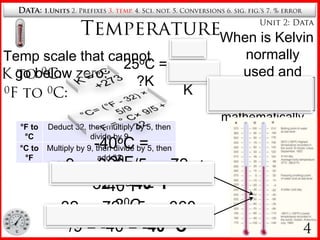
3. Temperature scales including Kelvin and conversions between Celsius, Fahrenheit and Kelvin.
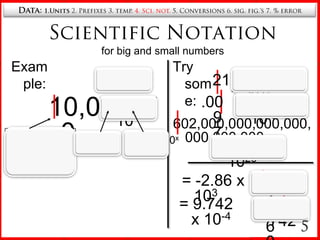
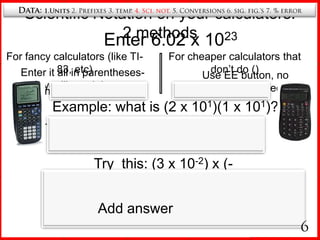
4. Scientific notation for writing very large and small numbers.
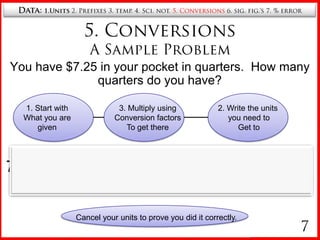
5. Converting between units using conversion factors and canceling units.
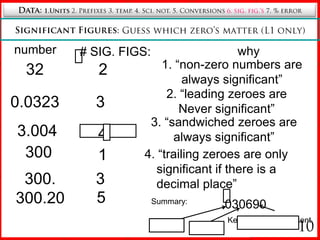
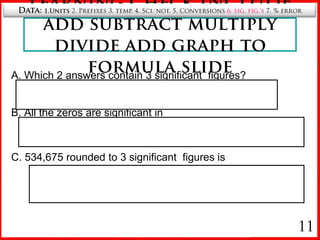
6. Rules for determining significant figures and counting zeros.
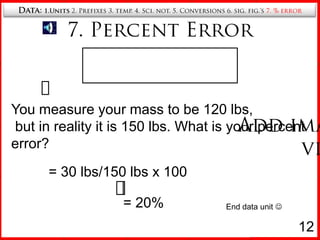
7. Calculating percent error in measurements.