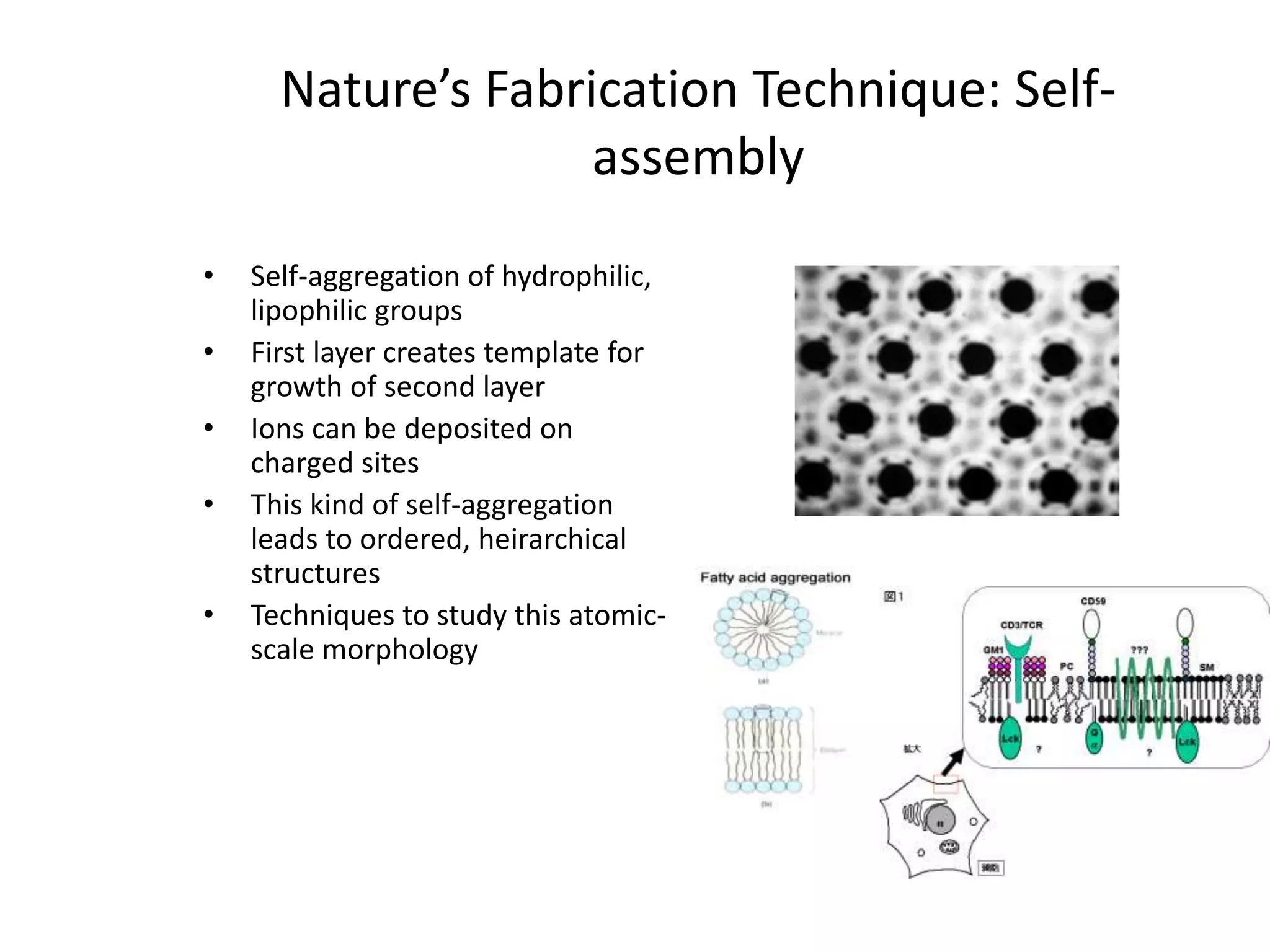
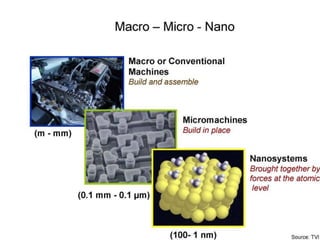
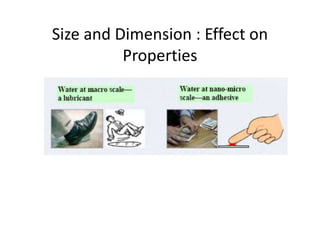
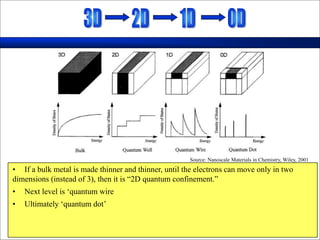
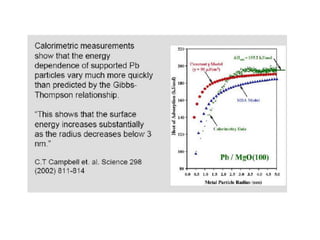
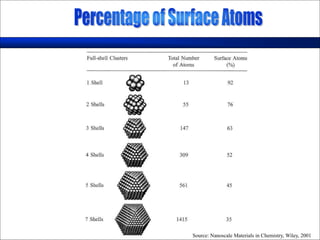
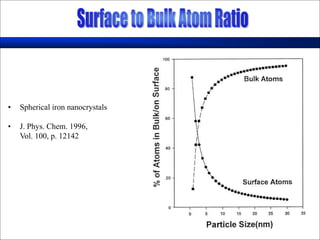
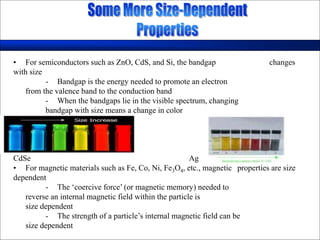
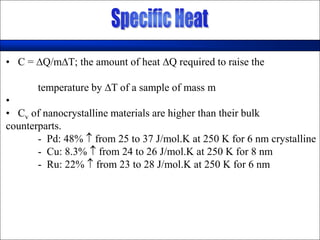
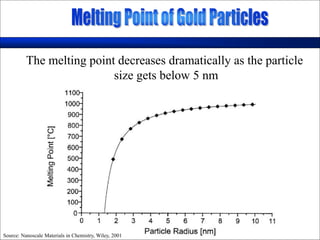
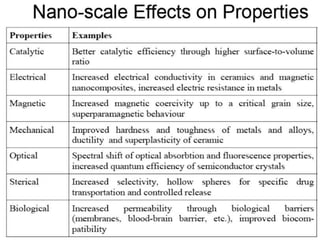
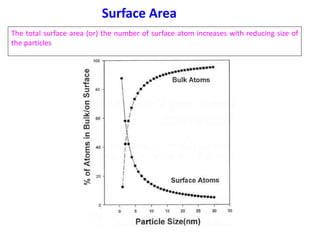
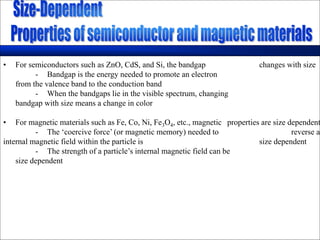
Nature uses self-assembly techniques to create ordered hierarchical structures at the atomic scale. When materials are reduced to the nanoscale, their properties change and are dependent on size. As materials get thinner, electrons become confined to two dimensions and then one, and at the smallest scale properties are dependent on quantum effects. Reducing the size of semiconductors alters their bandgap and therefore color, and reducing the size of magnetic materials changes their coercivity and magnetic fields. Nanoscale materials also demonstrate size-dependent increases in electrical conductivity, strength, and heat capacity compared to bulk materials.