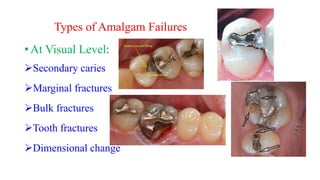
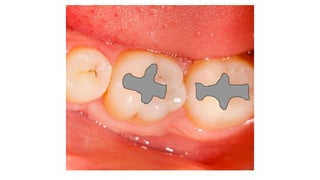
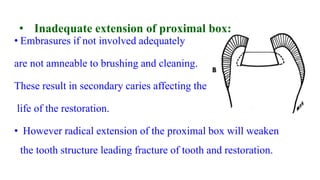
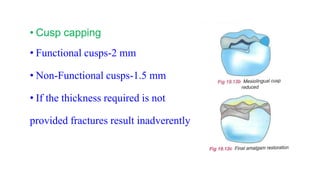
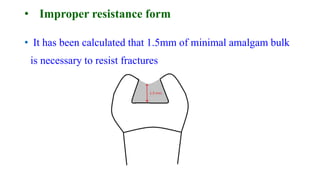
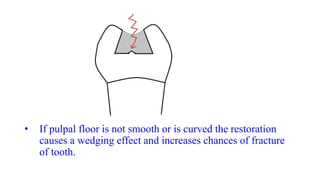
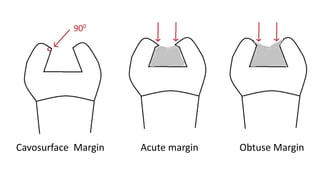
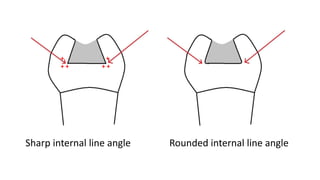
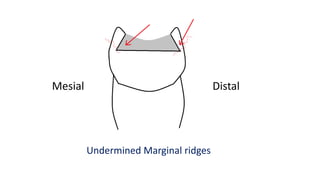
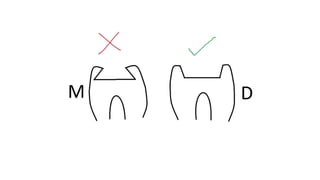
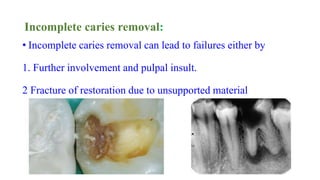
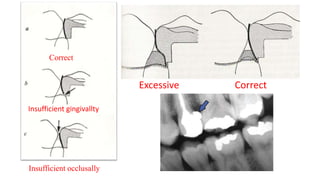
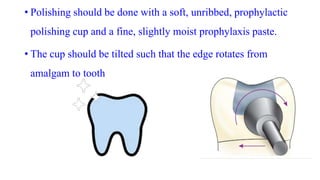
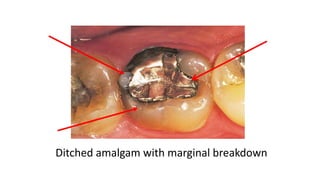
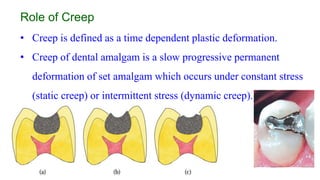
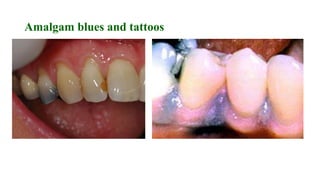
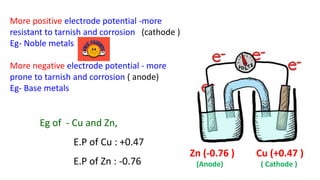
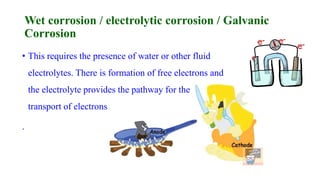
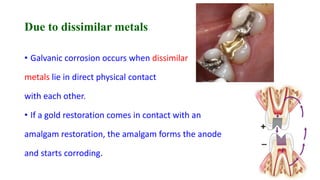
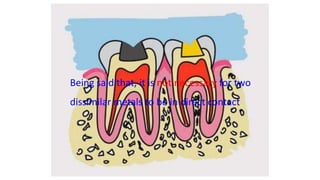
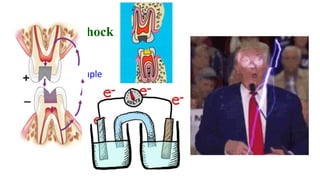
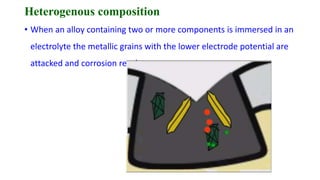
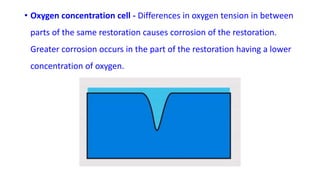
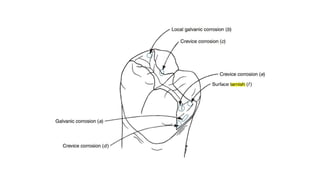
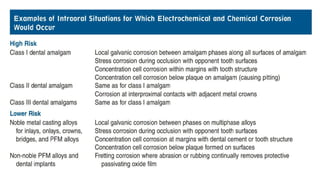
The document discusses the failures associated with silver amalgam as a restorative dental material, citing various causes such as faulty cavity preparation, improper manipulation, and contamination. It outlines different types of failures at visual and microstructural levels, emphasizing the importance of adequate preparation and condensation techniques. Additionally, it highlights the effects of corrosion and tarnish on amalgam restorations, concluding that proper manipulation can lead to successful outcomes.