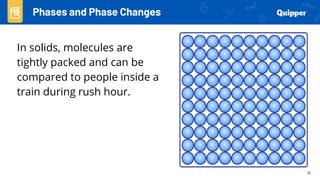
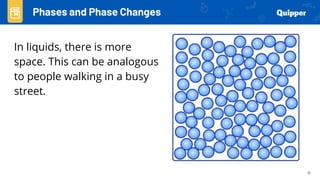
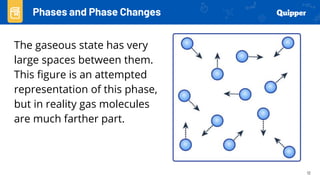
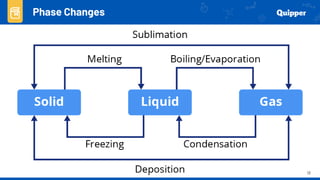
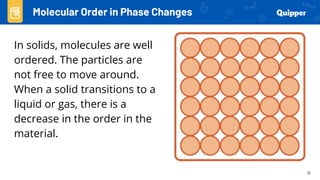
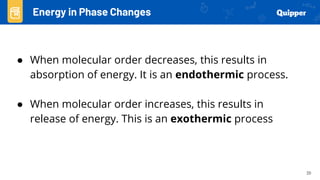
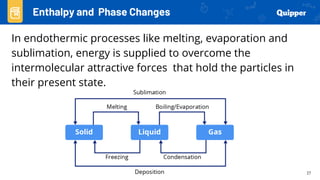
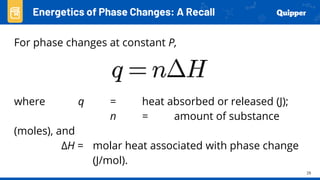
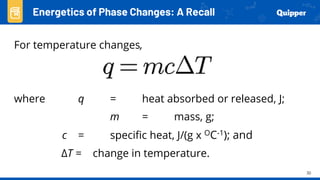
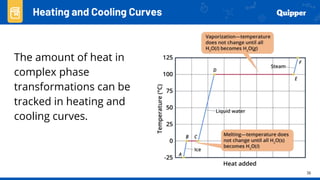
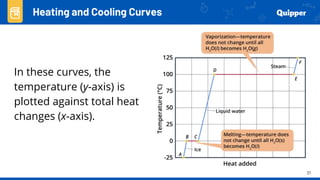
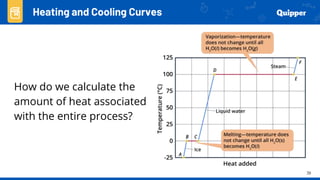
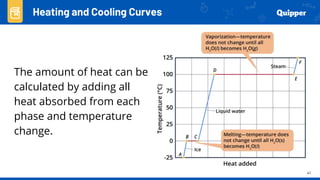
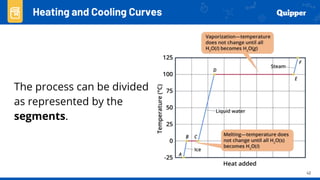
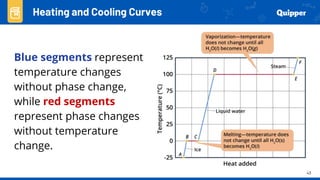
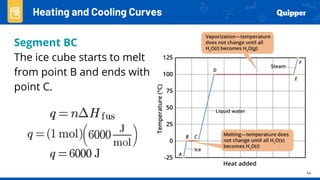
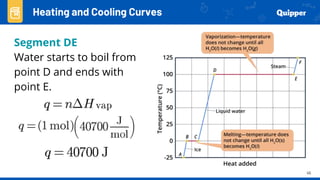
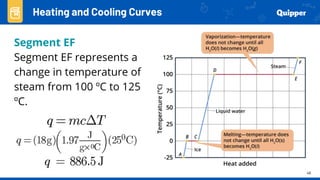
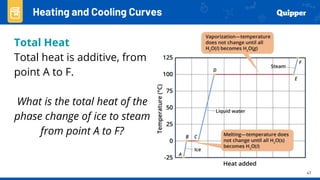
This document provides an overview of phase changes and energy changes that accompany phase changes. It discusses the molecular order in different phases of matter (solid, liquid, gas) and how phase changes result in an increase or decrease of molecular order. Phase changes that decrease molecular order are endothermic and require energy input, while those that increase order are exothermic and release energy. Heating and cooling curves are introduced to track temperature and energy changes during complex phase change processes. Formulas are provided to calculate energy for temperature changes and phase changes. Examples are given to practice these calculations.