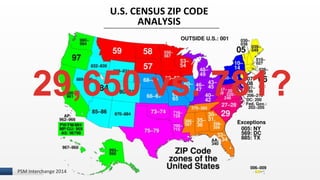
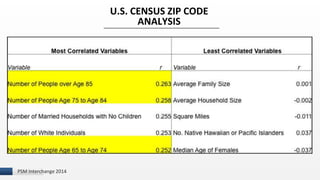
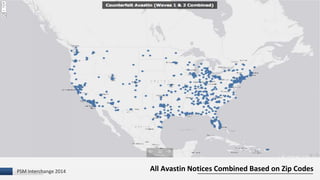
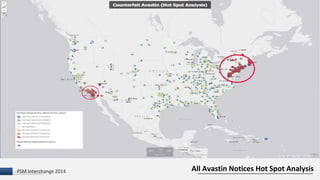
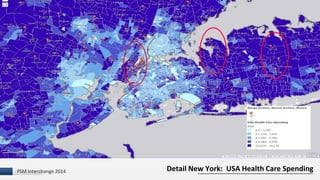
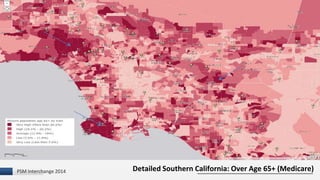
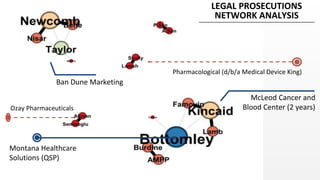
The document discusses the issue of counterfeit Avastin within the U.S. drug supply chain, highlighting key findings from a study on the prevalence and implications of counterfeit medications. It reports extensive FDA safety notices, legal prosecutions, and emphasizes the need for improved surveillance and education regarding counterfeit drugs. Additionally, it raises concerns about patient awareness and the challenges of tracking the impact of these counterfeit products on public health.