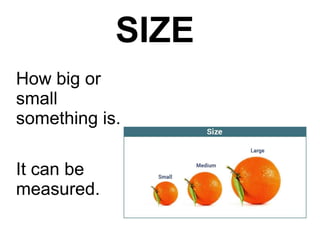
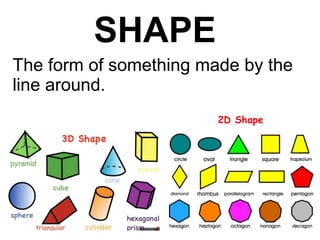
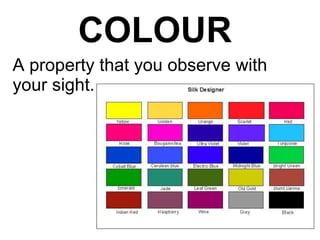
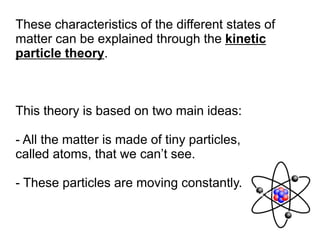
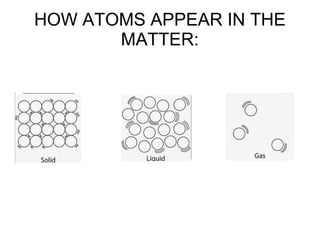
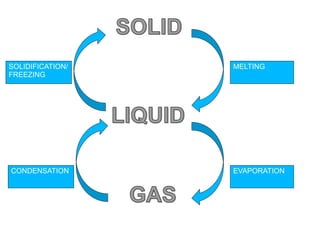
This document is about the properties and states of matter. It defines matter as anything that has mass and takes up space. There are three main states of matter: solids, liquids, and gases. Solids have a definite shape and volume, liquids have a definite volume but not shape, and gases have no definite shape or volume. The document explains the kinetic particle theory, which says matter is made of atoms that are constantly in motion, and how this explains the behaviors of the different states. It also discusses physical changes, like melting and boiling, which involve changes in state but no new substances, and chemical changes, which create new products and are typically irreversible.