potentiometry and it's use in titrations.pdf
•
0 likes•15 views
Potentiometry involves measuring electrode potentials across electrochemical cells to perform electrochemical analysis. An electrochemical cell consists of two half-cells connected by a salt bridge. In one half-cell, oxidation occurs, generating electrons that travel through the salt bridge to the other half-cell, where reduction occurs. The potential difference between the indicator and reference electrodes relates to the analyte concentration based on the Nernst equation and can be used to determine concentration. Common applications of potentiometry include clinical analysis, environmental analysis, and potentiometric titrations for accurate endpoint detection.
Report
Share
Report
Share
Download to read offline
Recommended
More Related Content
Similar to potentiometry and it's use in titrations.pdf
Similar to potentiometry and it's use in titrations.pdf (20)
Master Thesis Total Oxidation Over Cu Based Catalysts

Master Thesis Total Oxidation Over Cu Based Catalysts
Cyclic Voltammetry: Principle, Instrumentation & Applications

Cyclic Voltammetry: Principle, Instrumentation & Applications
Option C Nernst Equation, Voltaic Cell and Concentration Cell

Option C Nernst Equation, Voltaic Cell and Concentration Cell
Oxidation and Reduction (Uses of Redox potential data) By Latish Barve.pdf

Oxidation and Reduction (Uses of Redox potential data) By Latish Barve.pdf
Recently uploaded
+971581248768 Mtp-Kit (500MG) Prices » Dubai [(+971581248768**)] Abortion Pills For Sale In Dubai, UAE, Mifepristone and Misoprostol Tablets Available In Dubai, UAE CONTACT DR.Maya Whatsapp +971581248768 We Have Abortion Pills / Cytotec Tablets /Mifegest Kit Available in Dubai, Sharjah, Abudhabi, Ajman, Alain, Fujairah, Ras Al Khaimah, Umm Al Quwain, UAE, Buy cytotec in Dubai +971581248768''''Abortion Pills near me DUBAI | ABU DHABI|UAE. Price of Misoprostol, Cytotec” +971581248768' Dr.DEEM ''BUY ABORTION PILLS MIFEGEST KIT, MISOPROTONE, CYTOTEC PILLS IN DUBAI, ABU DHABI,UAE'' Contact me now via What's App…… abortion Pills Cytotec also available Oman Qatar Doha Saudi Arabia Bahrain Above all, Cytotec Abortion Pills are Available In Dubai / UAE, you will be very happy to do abortion in Dubai we are providing cytotec 200mg abortion pill in Dubai, UAE. Medication abortion offers an alternative to Surgical Abortion for women in the early weeks of pregnancy. We only offer abortion pills from 1 week-6 Months. We then advise you to use surgery if its beyond 6 months. Our Abu Dhabi, Ajman, Al Ain, Dubai, Fujairah, Ras Al Khaimah (RAK), Sharjah, Umm Al Quwain (UAQ) United Arab Emirates Abortion Clinic provides the safest and most advanced techniques for providing non-surgical, medical and surgical abortion methods for early through late second trimester, including the Abortion By Pill Procedure (RU 486, Mifeprex, Mifepristone, early options French Abortion Pill), Tamoxifen, Methotrexate and Cytotec (Misoprostol). The Abu Dhabi, United Arab Emirates Abortion Clinic performs Same Day Abortion Procedure using medications that are taken on the first day of the office visit and will cause the abortion to occur generally within 4 to 6 hours (as early as 30 minutes) for patients who are 3 to 12 weeks pregnant. When Mifepristone and Misoprostol are used, 50% of patients complete in 4 to 6 hours; 75% to 80% in 12 hours; and 90% in 24 hours. We use a regimen that allows for completion without the need for surgery 99% of the time. All advanced second trimester and late term pregnancies at our Tampa clinic (17 to 24 weeks or greater) can be completed within 24 hours or less 99% of the time without the need surgery. The procedure is completed with minimal to no complications. Our Women's Health Center located in Abu Dhabi, United Arab Emirates, uses the latest medications for medical abortions (RU-486, Mifeprex, Mifegyne, Mifepristone, early options French abortion pill), Methotrexate and Cytotec (Misoprostol). The safety standards of our Abu Dhabi, United Arab Emirates Abortion Doctors remain unparalleled. They consistently maintain the lowest complication rates throughout the nation. Our Physicians and staff are always available to answer questions and care for women in one of the most difficult times in their lives. The decision to have an abortion at the Abortion Clinic in Abu Dhabi, (United Arab Emirates)+971581248768+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...

+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...?#DUbAI#??##{{(☎️+971_581248768%)**%*]'#abortion pills for sale in dubai@
Recently uploaded (20)
300003-World Science Day For Peace And Development.pptx

300003-World Science Day For Peace And Development.pptx
Module for Grade 9 for Asynchronous/Distance learning

Module for Grade 9 for Asynchronous/Distance learning
Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b

Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b
Locating and isolating a gene, FISH, GISH, Chromosome walking and jumping, te...

Locating and isolating a gene, FISH, GISH, Chromosome walking and jumping, te...
Thyroid Physiology_Dr.E. Muralinath_ Associate Professor

Thyroid Physiology_Dr.E. Muralinath_ Associate Professor
Biogenic Sulfur Gases as Biosignatures on Temperate Sub-Neptune Waterworlds

Biogenic Sulfur Gases as Biosignatures on Temperate Sub-Neptune Waterworlds
Pests of mustard_Identification_Management_Dr.UPR.pdf

Pests of mustard_Identification_Management_Dr.UPR.pdf
Vip profile Call Girls In Lonavala 9748763073 For Genuine Sex Service At Just...

Vip profile Call Girls In Lonavala 9748763073 For Genuine Sex Service At Just...
High Profile 🔝 8250077686 📞 Call Girls Service in GTB Nagar🍑

High Profile 🔝 8250077686 📞 Call Girls Service in GTB Nagar🍑
+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...

+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...
Connaught Place, Delhi Call girls :8448380779 Model Escorts | 100% verified

Connaught Place, Delhi Call girls :8448380779 Model Escorts | 100% verified
potentiometry and it's use in titrations.pdf
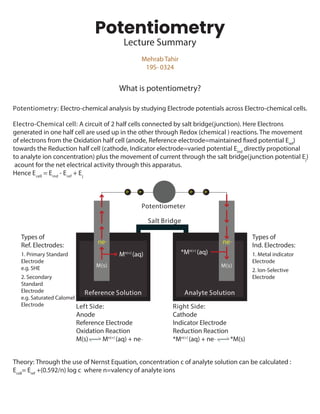
- 1. Mehrab Tahir 19S- 0324 Potentiometry Lecture Summary Potentiometry: Electro-chemical analysis by studying Electrode potentials across Electro-chemical cells. Electro-Chemical cell: A circuit of 2 half cells connected by salt bridge(junction). Here Electrons generated in one half cell are used up in the other through Redox (chemical ) reactions. The movement of electrons from the Oxidation half cell (anode, Reference electrode=maintained fixed potential Eref ) towards the Reduction half cell (cathode, Indicator electrode=varied potential Eind directly propotional to analyte ion concentration) plus the movement of current through the salt bridge(junction potential Ej ) acount for the net electrical activity through this apparatus. Hence Ecell = Eind - Eref + Ej Theory: Through the use of Nernst Equation, concentration c of analyte solution can be calculated : Ecell = Eref +(0.592/n) log c where n=valency of analyte ions Left Side: Anode Reference Electrode Oxidation Reaction M(s) Mn(+) (aq) + ne_ Right Side: Cathode Indicator Electrode Reduction Reaction *Mn(+) (aq) + ne_ *M(s) M(s) M(s) ne_ e_ e_ e_ e_ ne_ Mn(+) (aq) *Mn(+) (aq) Types of Ref. Electrodes: 1. Primary Standard Electrode e.g. SHE 2. Secondary Standard Electrode e.g. Saturated Calomel Electrode Types of Ind. Electrodes: 1. Metal indicator Electrode 2. Ion-Selective Electrode What is potentiometry? Potentiometer Salt Bridge Analyte Solution Reference Solution
- 2. Applications of potentiometry How do we perform potentiometric titrations? Clinical Chemistry: Clinical sample analysis (analyte conc. detected) Environmental Chemistry: Analysis of samples of air, water etc Potentiometric Titrations: pH change, end point detection with much accuracy Agriculture: Analysis of soil, fertilizers, plant materials. Food Processing: Analysis of food samples for nutrients/toxins Apparatus Setup: Precipitation Titration: M(s) ne_ *Mn(+) (aq) Potentiometer Titrate in burette 2H+ (aq) e_ e_ Titrand/ Analyte Solution H2 ne_ Ag(s) ne_ Ag+ (aq) Potentiometer halides e.g. Iodide, Chloride, Bromide etc 2H+ (aq) e_ e_ AgNO3 H2 ne_ AgNO3 + NaCl (limited as per titrand conc.) AgCl + NaNO3 At End Point: When all NaCl is used up, AgNO3 drop next added Ag+ conc. increases EMF spikes up EMF AgNO3 (ml) End Point Sharp EMF Rise observed at End Point SHE SHE
- 3. Acid-Base Titration: NaOH + HCl (limited as per titrand conc.) NaCl +H-OH At End Point: When all HCl is used up, NaOH drop next added OH- conc. increases pH spikes up pH NaOH (ml) pH Acidic pH Basic Sharp pH Rise observed at End Point End Point 1-Strong Acid- Strong Base Titration NH4 OH + HCl (limited as per titrand conc.) NH4 Cl + H-OH At End Point: When all HCl is used up, NH4 OH drop next added OH- conc. increase pH increases slightly 2-Strong Acid-Weak Base Titration pH Acidic pH Basic pH NH4 OH (ml) Slight pH Rise observed at End Point End Point pH Basic End Point pH Acidic NH4OH + Acetic Acid (limited as per titrand conc.) NH4-acetate + H-OH At End Point: When all acetic acid is used up, NH4 OH drop next added OH- conc. increase pH increases slightly pH NH4 OH (ml) Slight pH Rise observed at End Point 4-Weak Acid-Weak Base Titration pH Acidic pH Basic NaOH + Acetic Acid (limited as per titrand conc.) Na-acetate + H-OH At End Point: When all acetic acid is used up, NaOH drop next added OH- conc. increase pH increases pH NaOH (ml) pH Rise observed at End Point End Point 3-Weak Acid- Strong Base Titration
- 4. Much like the conventional Redox Potentiometry. Involves the transfer of electrons from the substance being oxidized to the substance being reduced. Oxidation/Reduction Titration: Complex Formation Titration: Cu(s) ne_ Cu+2 (aq) Potentiometer EDTA 2H+ (aq) e_ e_ CuSO4 H2 ne_ CuSO4 + EDTA (limited as per titrand conc.) Cu-EDTA Complex At End Point: When all EDTA is used up, CuSO4 drop next added Cu+2 conc. increases abrupt EMF changes SHE