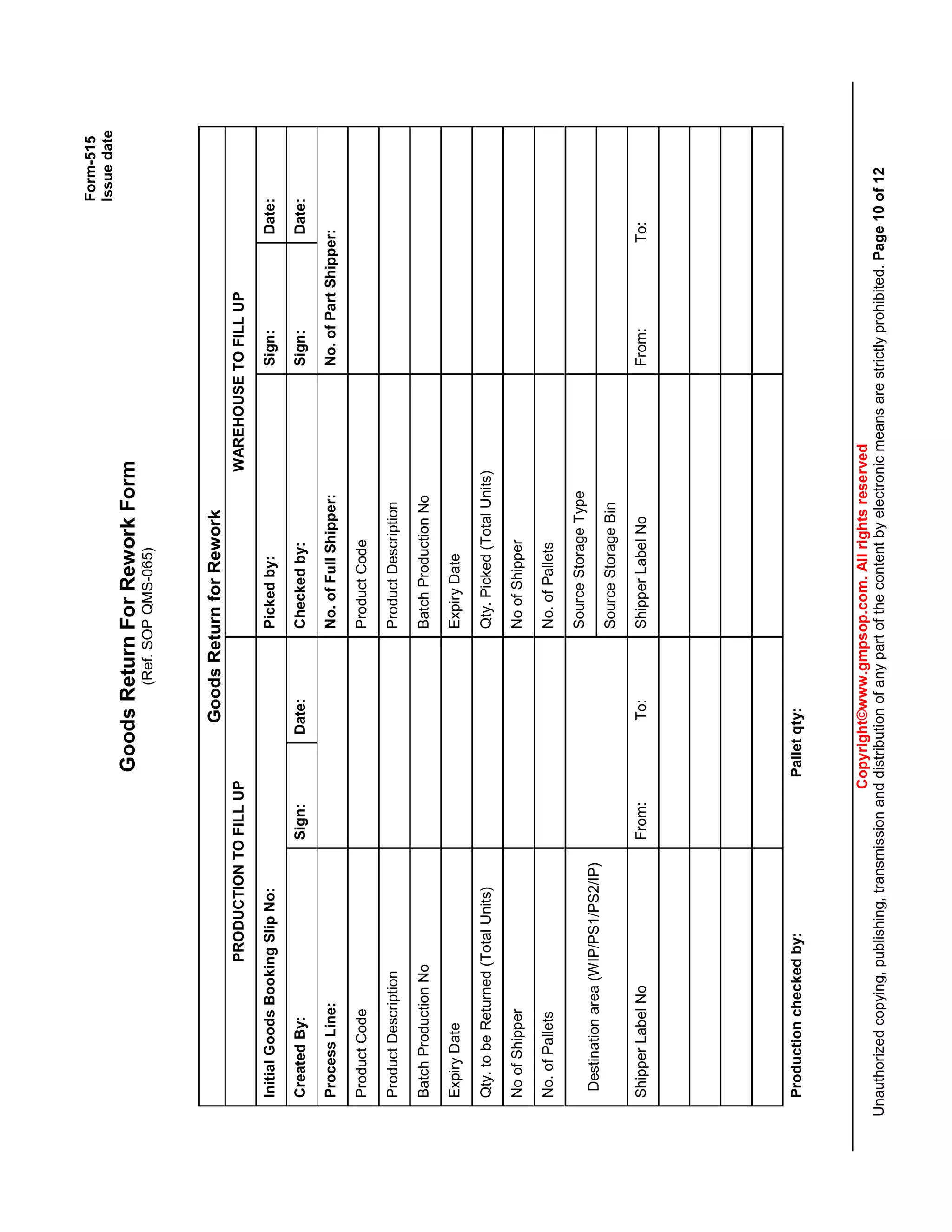
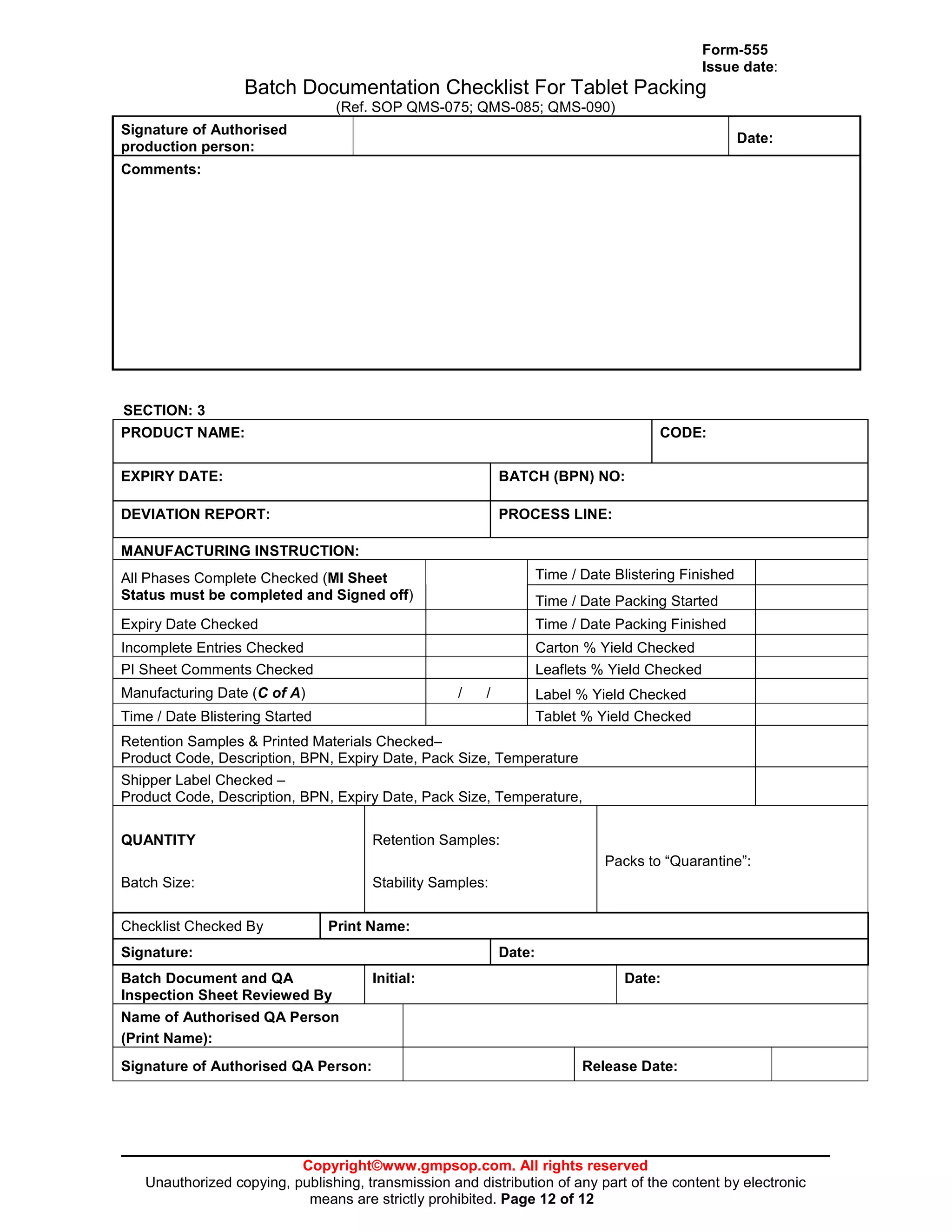
This document provides a standard operating procedure for reworking manufactured products. It outlines the process for generating a deviation report if a quality issue is identified, transferring products to a rework area, following an approved rework protocol, and documenting all rework activities. The procedure also addresses reworking products at a contract manufacturer and specifies templates for rework protocols.