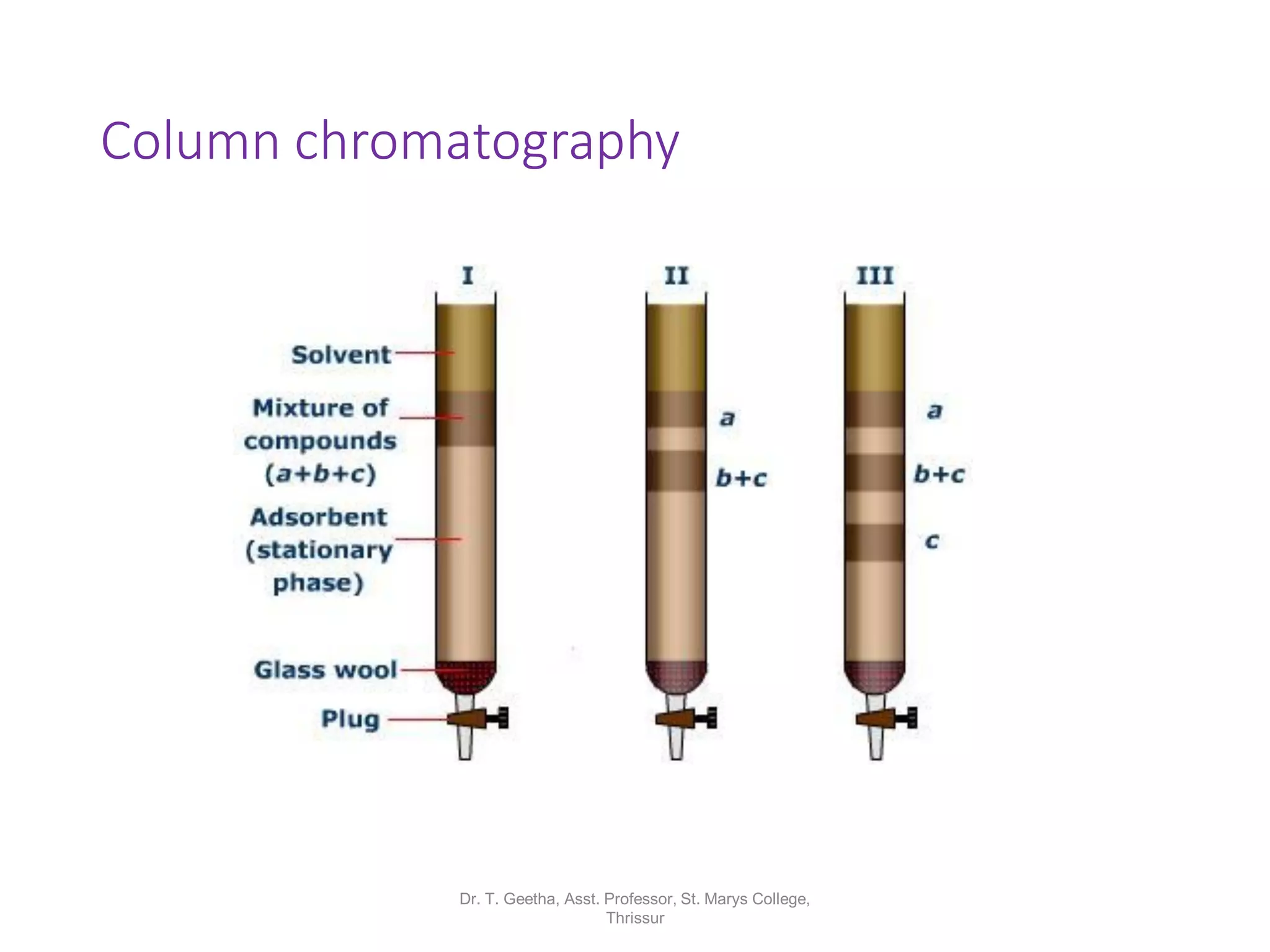
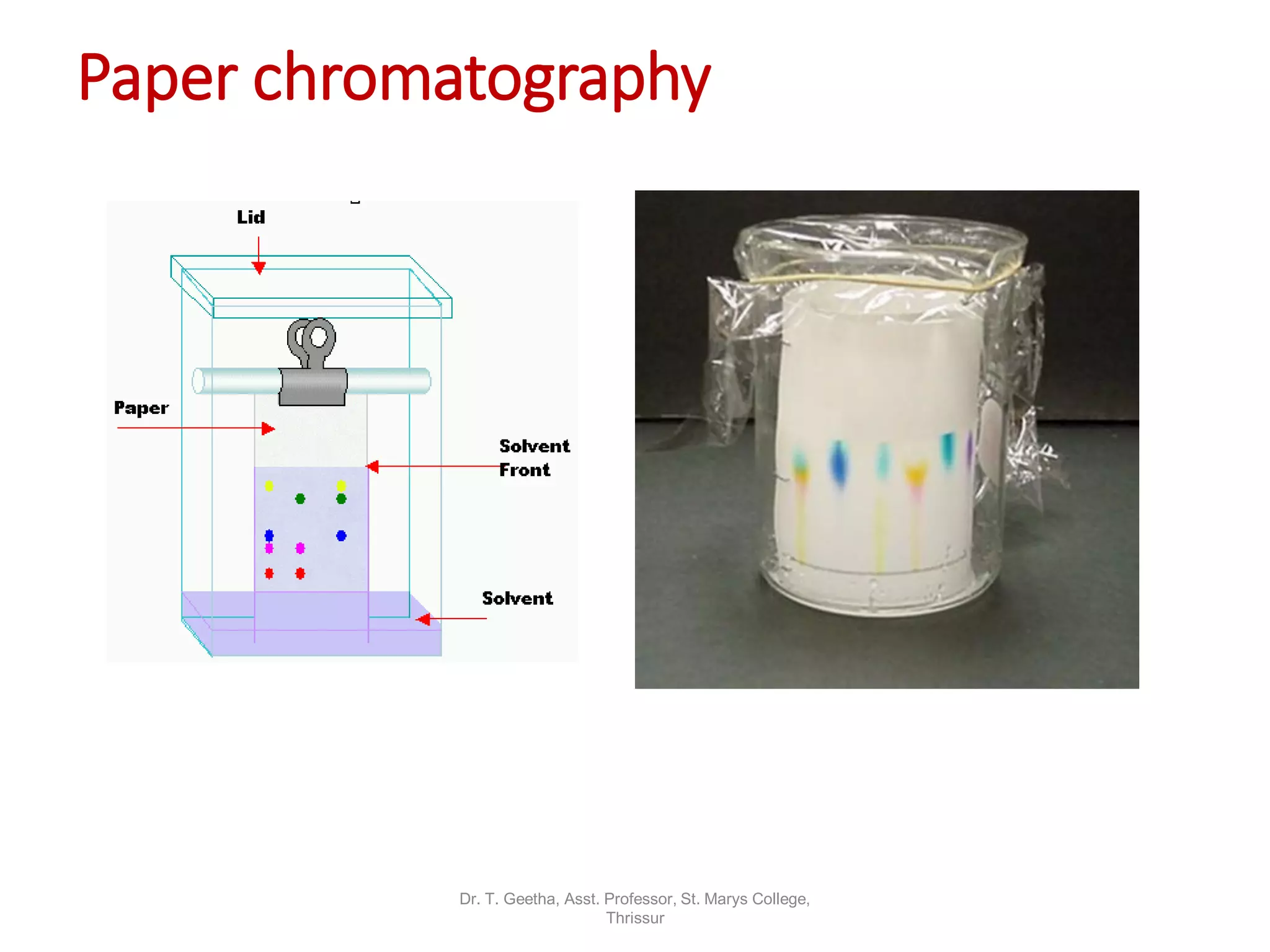
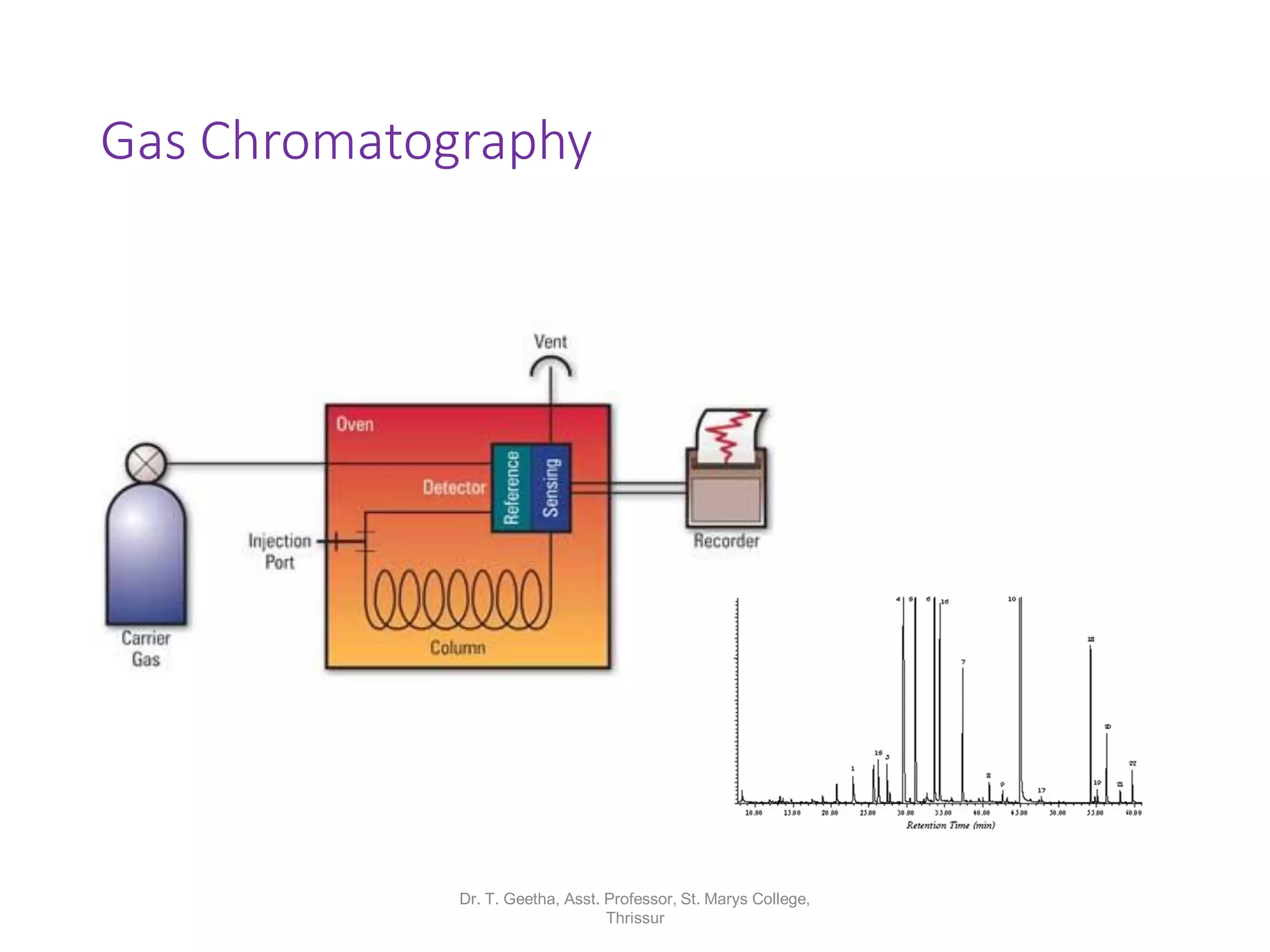
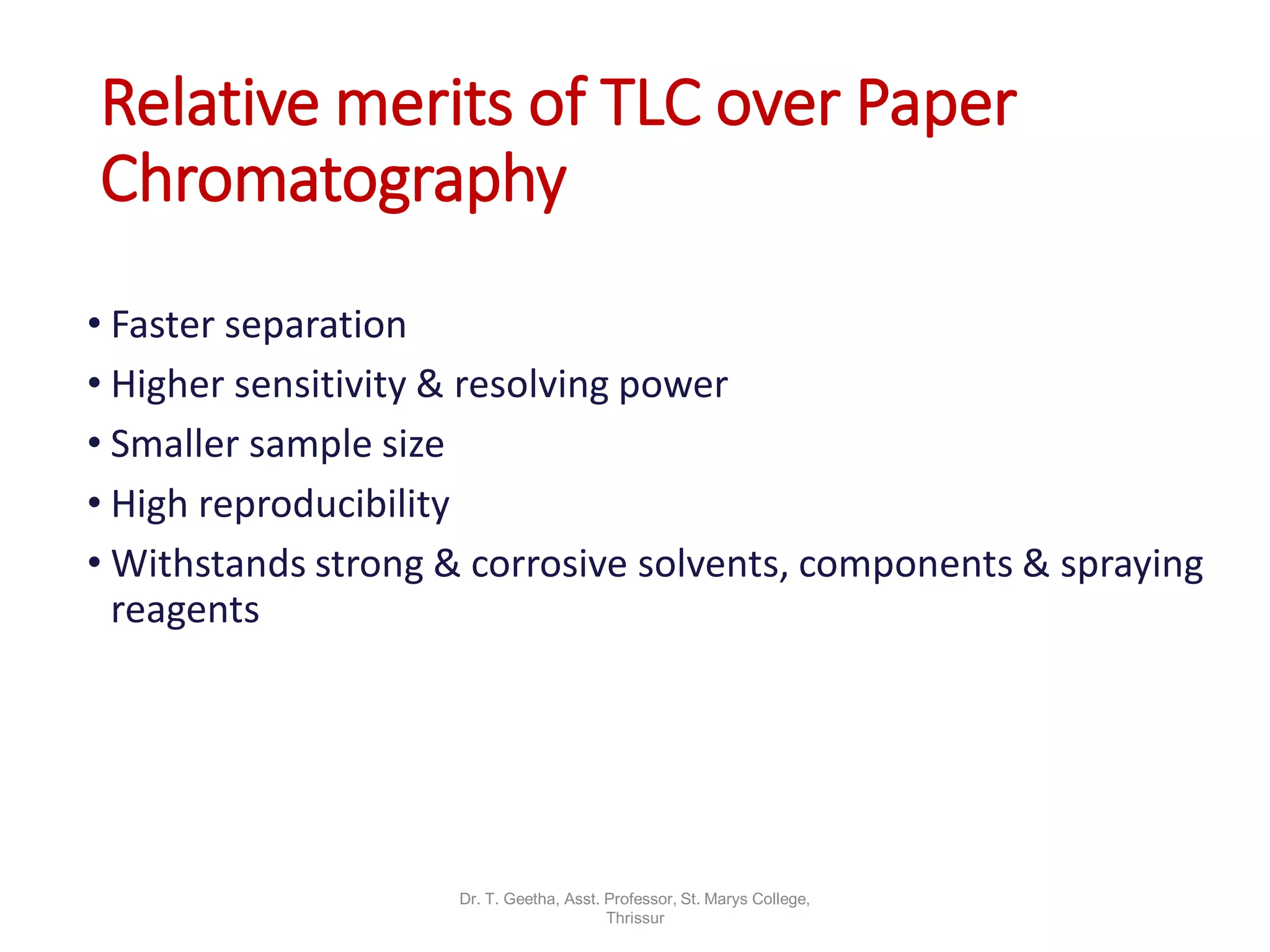
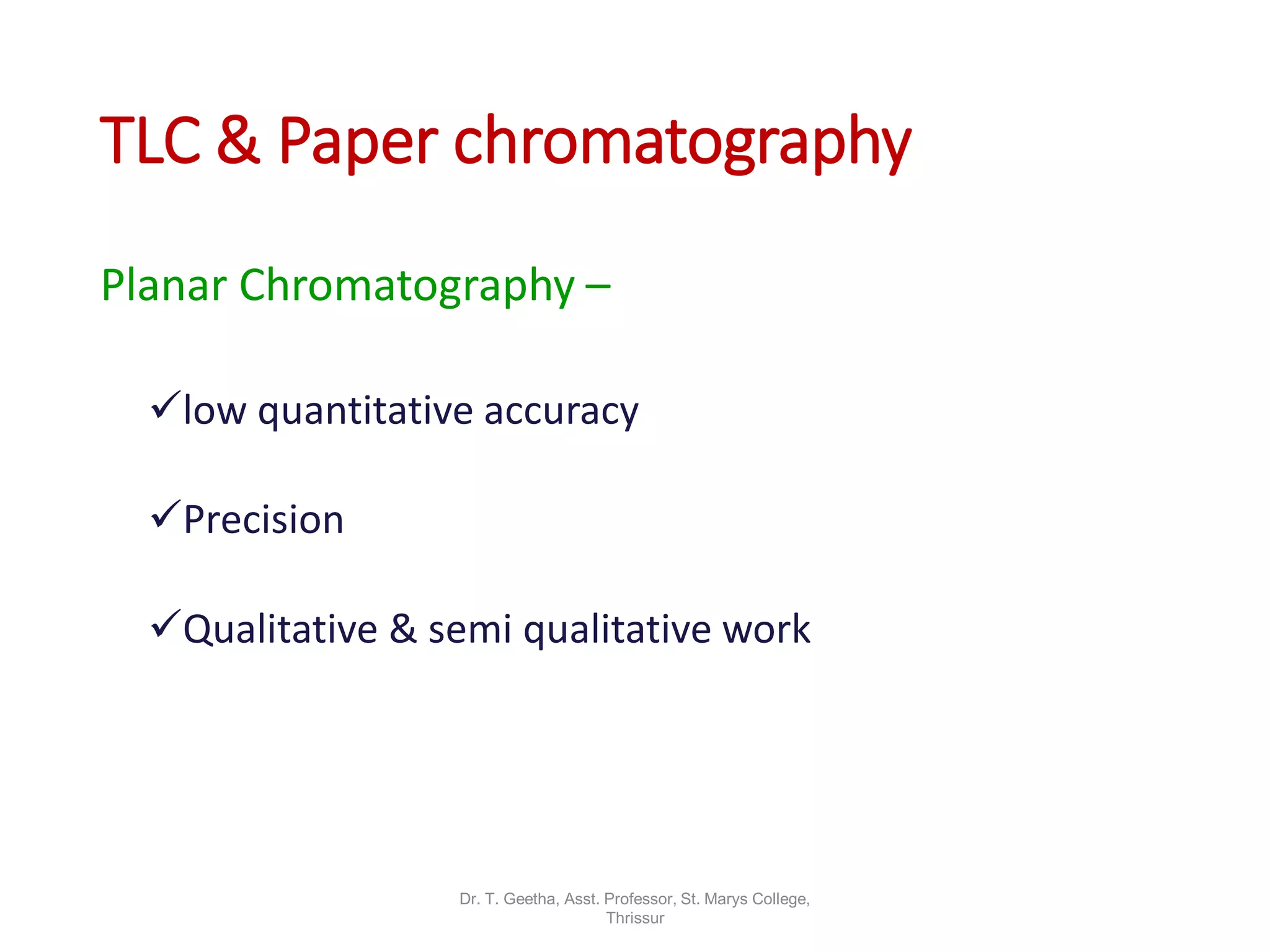
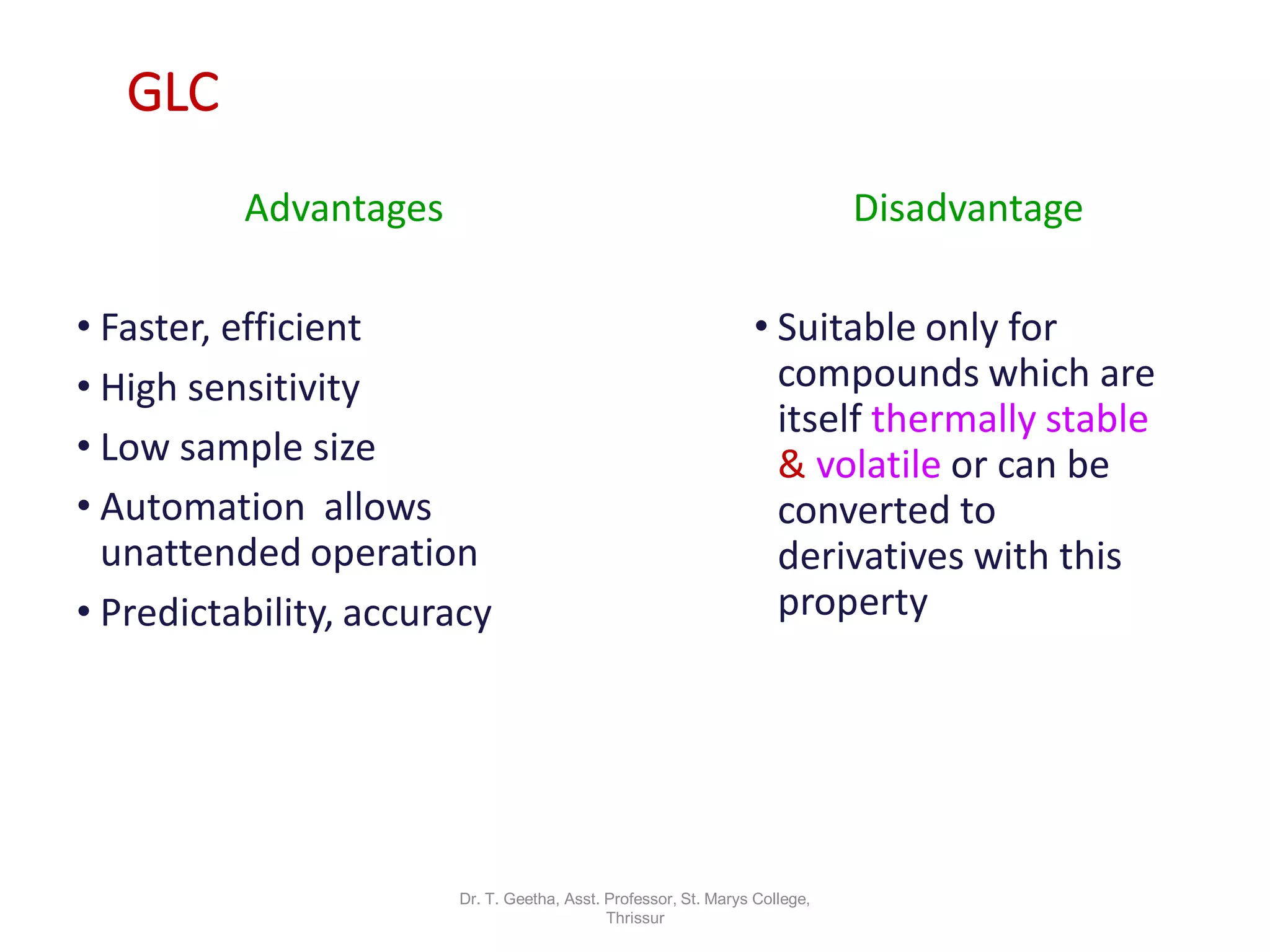
Chromatography is a technique used to separate mixtures by differential partitioning between two phases. There are several types including liquid-solid chromatography, gas-liquid chromatography, and thin layer chromatography. The document discusses the principles, procedures, and applications of various chromatography techniques like column chromatography, paper chromatography, thin layer chromatography, and gas chromatography. These techniques are used for separation, purification, and identification of mixtures in various fields like chemistry, biochemistry, medicine, food analysis, and more.