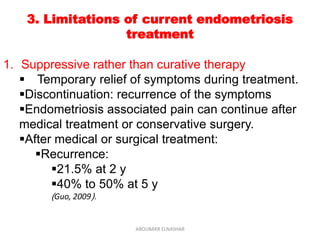
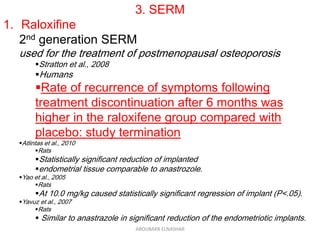
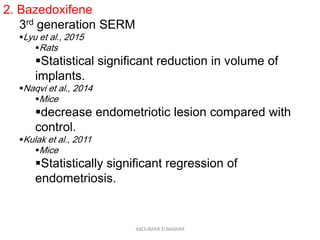
This document discusses emerging treatments for endometriosis. It begins by outlining the limitations of current treatments, such as being suppressive rather than curative, interfering with fertility, and having limited effectiveness for certain disease phenotypes. The document then examines the criteria for an ideal endometriosis medication and evaluates several emerging hormonal and non-hormonal treatments. These include gonadotropin-releasing hormone antagonists, selective progesterone receptor modulators, aromatase inhibitors, immunomodulators, and other agents. For many of the treatments, human and animal studies are summarized that demonstrate reductions in pain, lesion size, or other beneficial outcomes for endometriosis.