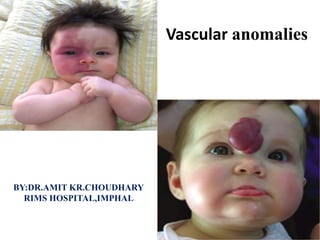
Vascular anomalies
- 2. Introduction • Vascular anomalies is a newly evolved field that incorporates several surgical and medical specialties. • For centuries, it was believed that vascular birthmarks were imprinted on the unborn child by a mother’s emotions or diet. This was reflected in words for brightly colored foods to describe vascular anomalies. Adjectives such as “cherry,” “strawberry,” and “port-wine” have their roots in these traditional beliefs. • Physicians usually preferred the Latin term naevus maternus for vascular birthmarks
- 3. • In the 19th century, the first attempt was made to categorize vascular anomalies histologically by Virchow, the father of cellular pathology. • Virchow’s angioma simplex became synonymous with “capillary” or “strawberry” hemangioma. His term angioma cavernosum was used indiscriminately for subcutaneous hemangiomas (that regress) and venous malformations (that never regress). • Angioma racemosum was modified to racemose (cirsoid) aneurysm or “arteriovenous hemangioma”, referring to an arteriovenous malformation, a vascular lesion that expands over time. • Wegener, developed a comparable histomorphic subcategorization for “lymphangioma • This confusing nosology has been responsible for improper diagnosis, illogical treatment, and misdirected research.
- 4. • A biologic classification system, introduced in 1982,cleared the terminologic confusion that had long obscured the field. This scheme evolved from studies that correlated physical findings, natural history, and cellular features. • The key to this biologic classification is proper use of the Greek nominative suffix -oma which once meant “swelling” or “tumor”. In modern times -oma denotes a lesion that arises by upregulated cellular growth.
- 6. • Biologic classification was accepted by the International Society for Vascular Anomalies in 1996. • Differences that distinguish between hemangiomas and vascular malformations were confirmed by imaging and by immunohistochemical markers. • vascular malformations, although fundamentally structural disorders, can exhibit endothelial hyperplasia, possibly triggered by clotting, ischemia, embolization, partial resection, or hormonal influences. • History and physical examination should give diagnostic accuracy of more than 90% in distinguishing between vascular tumors and vascular malformations • The most likely error in assigning a clinical diagnosis continues to be an inaccurate, imprecise use of terminology , Perhaps the most egregious example is “hemangioma”, so often applied generically and indiscriminately to vascular lesions that are entirely different in histology and behavior. • There is no such entity as “cavernous hemangioma”. The lesion is either a deep infantile hemangioma or a venous malformation.
- 7. Vascular tumors • Vascular tumors are endothelial neoplasms characterized by increased endothelial turnover. • Infantile hemangioma is the most common, a tumor that arises in infants. • Other vascular tumors are congenital hemangioma, hemangioendotheliomas, tufted angioma, hemangiopericytomas, angiosarcoma, pyogenic granuloma.
- 8. Vascular malformations • Vascular malformations are the result of abnormal development of vascular elements during embryogenesis . They are designated according to the predominant channel type as: capillary malformation, lymphatic malformation, venous malformation, arteriovenous malformation, and complex forms such as capillary- lymphatic-venous malformation.
- 11. Vascular tumors
- 12. Infantile hemangioma Pathogenesis Infantile hemangioma (IH) is a benign endothelial tumor with a biologic behavior that is unique because it grows rapidly, slowly regresses, and never recurs.
- 13. There are three stages in its life cycle: The proliferating phase (0–1 year of age) The involuting phase (1–4 years of age) The involuted phase (after 4 years of age).
- 14. During the proliferating phase, the histopathologic examination shows clusters of plump endothelial cells with small vascular channels and minimal connective tissue. During involution, mature blood vessels are formed. Vascular channels enlarge and are lined by flattened endothelial cells. Increased extracellular matrix, multi-laminated basement membranes, and pericytes are deposited around the vessels. After involution, the majority of the IH is replaced with adipocytes andconnective tissue. All that remains are thin- walled vessels with multi-laminated basement membranes and larger feeding and draining vessels
- 15. Clinical features 4–5% of Caucasian infants. more frequent in premature children and in females (4 : 1). The tumor is typically single (80%) and involves the head and neck (60%), trunk (25%), or extremity (15%). The median age of appearance is 2 weeks. First 9 months of age IH grows faster than the child (proliferating phase); IH is red when it involves the superficial dermis. By age 9–12 months, growth of IH reaches a plateau. After 12 months, the tumor begins to regress (involuting phase); the color fades and the lesion flattens. Involution ceases most of children by age 4 years (involuted phase).
- 16. Head and neck hemangiomas Ulcerated lesions may destroy the eyelid, ear, nose or lip. IH of the scalp or eyebrow can result in alopecia. Periorbital hemangioma can block the visual axis or distort the cornea causing amblyopia. Subglottic hemangioma may obstruct the airway. Multiple hemangiomas Approximately 20% of infants have more than one IH. The term hemangiomatosis designates five or more small (<5 mm) tumors. These children are more likely to have IH of internal organs. The liver is most commonly affected.
- 17. Hepatic hemangiomas • The liver is the most common extracutaneous site for IH. • There are three subtypes of hepatic hemangioma: focal, multifocal, or diffuse. • Most hepatic IHs are nonproblematic and large tumors can cause heart failure, hepatomegaly, anemia, or hypothyroidism. • Focal hepatic hemangioma is usually asymptomatic and not associated with cutaneous lesions.
- 18. Hemangiomas and structural anomalies • PHACE association affects 2.3% of patients with IH, and consists of a plaque-like IH in a regional distribution of the face with at least one of the following anomalies: Posterior fossa brain malformation; Hemangioma; Arterial cerebrovascular anomalies; Coarctation of the aorta and cardiac defects; Eye/Endocrine abnormalities. • When ventral developmental defects (Sternal clefting or Supraumbilical raphe) are present, an “S” is added (PHACES).
- 19. Diagnosis • History and physical examination. • Hand-held Doppler- Fast-flow is confirmed by using this device. • Ultrasonography, IH appears as a soft- tissue mass with fast-flow, decreased arterial resistance, and increased venous drainage. • MRI, the tumor is isointense on T1, hyperintense on T2, and enhances during the proliferating phase. Involuting IH exhibits increased lobularity and adipose tissue; the number of vessels and flow is reduced. • Biopsy is indicated if malignancy is suspected • If biopsy is needed, positive erythrocyte- type glucose transporter (GLUT 1) immunostaining differentiates IH from other vascular tumors and malformations. Tumors or fast-flow lesions that may be confused with IH include: arteriovenous malformation, congenital hemangiomas, cutaneous leukemia (chloroma), hemangioendotheliomas, infantile fibrosarcoma, infantile myofibromatosis, lymphoma, metastatic neuroblastoma, PTEN-associated vascular anomaly, and pyogenic granuloma
- 20. Management
- 21. Nonoperative management Observation • 90% IH are small, localized, and do not involve anatomically important areas. • Monthly basis, during the proliferative phase if a lesion has the potential to cause obstruction, destruction, or ulceration requiring intervention. • Annually during the involuting phase if it is possible surgical intervention may be necessary in childhood for excess skin, residual fibrofatty tissue, or reconstruction of damaged structures.
- 22. Wound care • Superficial IH is prone to ulceration because the skin is damaged by the tumor. In addition, arteriovenous shunting reduces oxygen delivery to the skin, causing ischemia. • IH should be kept moist with hydrated petroleum during the proliferative phase. • Anogenital area may be further protected by using a petroleum gauze. • If ulceration develops, application of topical antibiotic ointment and petroleum gauze barrier. • Large, deep ulcers require damp-to-dry dressing changes. • EMLA (eutectic mixture of local anesthetics) contains prilocaine should not be used in infants less than three months of age because of the risk of methemoglobinemia.
- 23. Topical corticosteroid • Adverse effects include hypopigmentation, cutaneous atrophy, and even adrenal suppression. Intralesional corticosteroid • Small, well-localized IHs that obstruct the visual axis or nasal airway, or those at risk for damaging important structures (i.e., eyelid, lip, nose) are best managed by intralesional corticosteroid . • Triamcinolone (3 mg/kg) stabilizes the growth of the lesion.
- 24. Systemic pharmacotherapy • Oral prednisolone or propranolol - Problematic IH that is larger than 3–4 cm in diameter is managed by. • Interferon - Not recommended in children less than 12 months of age because it can cause neurologic sequela, particularly spastic diplegia. • Vincristine - as a second-line option. • prednisolone 3 mg/ kg/day for one month; then tapered by 0.5 cc every 2-4 weeks until it is discontinued between 10-12 months of age when the tumor is no longer proliferating.
- 26. Embolic therapy • Large IH, usually multifocal hepatic lesions. • Cardiac failure often recurs even after initial improvement, and drug therapy should be continued after embolization until the child is approximately 10– 12 months of age when natural involution begins. Laser therapy • pulsed-dye laser treatment • The laser penetrates only 0.75– 1.2 mm into the dermis, and thus only affects the superficial portion of the tumor. • Risk of ulceration, pain, bleeding, and scarring. • pulsed-dye laser is indicated during the involuted phase to fade residual telangiectasias.
- 27. Operative management Proliferative phase (infancy) • Resection of IH generally is not recommended during the early growth phase. The tumor is highly vascular during this period and there is a risk for blood loss, iatrogenic injury, and an inferior outcome, compared to excising residual tissue after the tumor has regressed. Indications for operative intervention during this phase: failure or contraindication to pharmacotherapy; well-localized tumor in an anatomically favorable area; If resection will be necessary in the future and the scar would be the same , Circular lesions located in visible areas, particularly the face, are best removed by circular excision and purse-string closure.
- 28. Involuting phase (early childhood) • Resection during involution is much safer . • Staged or total excision should be considered during this period, rather than waiting for complete involution if: (1) it is clear that the lesion will require resection (e.g., post- ulceration scarring, destroyed structures, expanded skin, significant fibrofatty residuum); (2) the length of the scar would be similar if the procedure was postponed to the involuted phase; (3) the scar is in a favorable location.
- 30. Involuted phase (late childhood) • Waiting until IH has fully involuted prior to removal ensures that the least amount of fibrofatty residuum and excess skin is resected, resulting in the smallest possible scar. • Allowing for full involution is recommended for lesions when it is unclear if a surgical scar would leave a worse deformity than the appearance of the residual hemangioma
- 33. Clinical features • There are rare hemangiomas that arise in the fetus. • Fully-grown at birth, and do not have postnatal growth. • Red violaceous with coarse telangiectasias, central pallor, and a peripheral pale halo. • More common in the extremities, have an equal sex distribution, • Solitary with an average diameter of 5 cm. • There are two forms: Rapidly involuting congenital hemangioma (RICH) and Noninvoluting congenital hemangioma (NICH).
- 34. RICH involutes rapidly after birth and 50% of lesions have completed regression by 7 months of age; the remaining tumors are fully involuted by 14 months. RICH affects the head or neck (42%), limbs (52%) or trunk (6%). RICH does not leave behind a significant adipose component, unlike common IH. NICH, in contrast, does not regress; it remains unchanged with persistent fast-flow.It involves the head or neck (43%), limbs (38%), or trunk (19%).
- 35. Management • RICH - usually does not require resection in infancy because it regresses so quickly. • congestive heart failure, and this is controlled by corticosteroid or embolization as the lesion involutes. • RICH may leave behind atrophic skin and subcutaneous tissue. • Reconstruction with autologous grafts (fat, dermis) or acellular dermis may be indicated. • NICH is rarely problematic in infancy; it is observed until the diagnosis is clear. Resection of NICH may be indicated to improve the appearance of the affected area, as long as the surgical scar will be less noticeable than the lesion.
- 37. Clinical features • Kaposiform hemangioendothelioma (KHE) is a rare vascular neoplasm that is locally aggressive, but does not metastasize. • Although one-half of lesions are present at birth, KHE may develop during infancy (58%), between age 1–10 years (32%), or after 11 yearsof age (10%);adult-onset is rare.
- 38. • KHE has an equal sex distribution, is solitary, and affects the head/neck (40%), trunk (30%), or an extremity (30%). • The tumor is often >5 cm in diameter and appears as a flat, reddish purple, edematous . • Over half the patients have Kasabach–Merritt phenomenon (KMP) (thrombocytopenia <25 000/mm3, petechiae, bleeding).
- 39. DIAGNOSIS • History, physical examination, and imaging. • MRI is indicated for diagnostic confirmation and to asses the extent of the tumor. • Histologically, KHE has infiltrating sheets or nodules of endothelial cells lining capillaries. • Hemosiderin-filled slitlike vascular spaces with red blood cell fragments, as well as dilated lymphatics, are present.
- 40. Management • Patients with KMP require systemic treatment to prevent life threatening complications. • Large, asymptomatic tumors without KMP are also managed with pharmacotherapy to minimize fibrosis and subsequent long-term pain and stiffness. • Vincristine is first-line therapy, the response rate is 90%. • interferon (50%) or corticosteroid (10%) are second line drugs.
- 41. Pyogenic granuloma • Pyogenic granuloma (PG) is neither “pyogenic” nor “granulomatous”. Some pathologists call it lobular capillary hemangioma. • PG is a solitary, red papule that grows rapidly on a stalk. It is small, with an average diameter of 6.5 mm; the mean age of onset is 6.7 years. • The male to female ratio is 2 : 1. • PG is commonly complicated by bleeding (64%) and ulceration (36%).
- 42. • PG primarily involves the skin (88%), but can also involve mucous membranes (11%). • It is distributed on the head or neck (62%), trunk (19%), upper extremity (13%), or lower extremity (5%). • PG rarely spontaneously heals. • PGs require intervention to control likely ulceration and bleeding. • Curettage, shave excision, laser therapy, and excision. • Full-thickness excision is more definitive treatment.
- 44. Capillary malformation Pathogenesis • Capillary malformation (CM) is the modern term for the antiquated “port- wine” stain. • The 19th century “neurovegetative theory” asserted that the primary embryonic defect occurs in the developing autonomic nervous system. • Regional or dermatomal (particularly with branches of the trigeminal nerve), suggesting a relationship to the developing peripheral nervous system.
- 45. • The cutaneous flush of a CM may, in part, be due to an inability of these vessels to constrict secondary to diminished sympathetic innervation. • The occasional finding of hyperhidrosis in an area of CM supports such an association.
- 46. Clinical features • CMs occur anywhere on the body; they can be localized or extensive. Rarely, they are multiple and generalized, such as in Sturge–Weber syndrome. • CM should not be confused with a nevus flammeus neonatorum, the most common vascular birthmark, seen in 50% of white neonates. These macular stains are popularly referred to as “angel kiss” on the forehead, eyelids, nose, and upper lip or “stork bite” in the nuchal area. • These predictably fade by 2 years of age, representing a minor transient dilatation of dermal vessels. If they persist, such a birthmark must be relabeled CM.
- 47. • Equal gender distribution; • The birth prevalence is 0.3%. • The cutaneous discoloration is usually, but not always, evident at birth because the stain may be hidden by the erythema of neonatal skin. • sometimes with raised fibrovascular cobblestoning. • Facial CMs often occur in a dermatomal distribution; 45% are restricted to one of the three trigeminal dermatomes. • Pyogenic granuloma may develop in CM, causing ulceration and bleeding. CM also can lead to soft-tissue and skeletal overgrowth below the stain. • When located on the face, hypertrophy of the lip, cheek, or forehead can occur; the lip is most commonly affected. • Enlargement of the maxilla or mandible can result in an occlusal cant (vertical maxillary overgrowth) with increased dental show and malocclusion.
- 48. • Increased circumference and limb length discrepancy. • A capillary stain on the posterior thorax can signify an underlying arteriovenous malformation of the spinal cord (Cobb syndrome). • A CM over the cervical or lumbosacral spine is a red flag for occult spinal dysraphism, lipomeningocele, tethered spinal cord, and diastematomyelia.
- 49. Management Pulse-dye laser therapy • Facial CM is best treated with pulse-dye laser early in childhood, before memory or self-awareness begins. • Intervention in infancy may achieve superior lightening of the lesion, as well as reduce the risk of subsequent darkening and hypertrophy, compared with photocoagulation in later childhood. • Multiple treatments, spaced 6 weeks apart, are often required until the CM fails to improve with additional treatments.
- 50. • Adulthood Cutaneous fibrovascular hypertrophy occurs over many years, requiring intervention in adulthood. • Malocclusion can be corrected in adolescence with orthodontic manipulation,orthognathic procedure,Le Fort I osteotomy or bimaxillary procedure. • Facial asymmetry caused by overgrowth of the zygoma, maxilla, or mandible can be improved by contour burring. • Trunk or extremity soft tissue overgrowth can be associated with increased subcutaneous adipose tissue. • Suction-assisted lipectomy. • Severe cutaneous thickening and cobblestoning can be resected and reconstructed by linear closure, skin grafts, or local flaps.
- 51. CUTIS MARMORATA TELANGIECTASIA CONGENITA • Cutis marmorata telangiectasia congenita (CMTC) manifests as congenital cutaneous marbling, even at normal temperatures, that becomes more pronounced with lower temperatures or with crying. • The involved skin is depressed in a serpiginous reticulated pattern and has a deep purple color.
- 52. • CMTC occurs sporadically in an equal gender distribution. • CMTC can cause ulceration and may be localized, segmental, or generalized. • It most frequently involves the trunk and extremities; it is typically unilateral (65%) and involves a lower extremity (69%). • The affected extremity is often hypoplastic. • Atrophy, pigmentation, and ectasia of the superficial veins often persist into adulthood. • CMTC may be associated with hypoplasia of the iliac and femoral veins.
- 54. Pathogenesis • The lymphatic system develops during the 6th week of embryonic life. • Paired jugular lymph sacs appear, followed by mesenteric, cisterna chyli, and posterior lymph sacs. These enlarge and become connected to the thoracic duct. • A second stage of maturation involves the transformation of the sacs into primary lymph nodes and the centrifugal spread of peripheral lymphatics. • One etiologic theory for LMs is that their sprouting lymphatic channels become “pinched off” from the main lymphatic system, leading to aberrant collections of lymphatic fluid-filled spaces. • Another theory attributes LMs to abnormal budding of the lymphatic system with a loss of connection to the central lymph channels or to development of lymphatic tissue in aberrant locations.
- 55. Clinical features • LM is characterized by the size of the malformed channels: microcystic, macrocystic, or combined. • Macrocystic lesions are defined as cysts large enough to be punctured by a needle and treated by sclerotherapy. • LM is usually noted at birth or within the first 2 years of life. • Prenatal ultrasonography can detect relatively large lesions as early as the 2nd trimester. • True LMs seen antenatally must be differentiated from “posterior nuchal translucency” or “cystic hygroma”.
- 56. • Most common locationis head and neck; other common sites are the axilla, chest, and perineum. • Lesions are soft and compressible. The overlying skin may be normal, have a bluish hue, or be studded with pink-red vesicles. Most common complications are bleeding and infection. Intralesional bleeding occurs in up to 35% of lesions causing ecchymotic discoloration, pain, or swelling. Infection complicates as many as 70% of lesions and can progress rapidly to sepsis.
- 57. • Oral lesions may lead to macroglossia • Two-thirds of infants with cervicofacial LM require tracheostomy
- 58. • Bony overgrowth is another complication; the mandible is most commonly involved resulting in an open bite and prognathism. • Thoracic or abdominal LM may lead to pleural, pericardial, or peritoneal chylous effusions. • Periorbital LM causes a permanent reduction in vision (40%), and 7% of patients become blind in the affected eye.
- 59. • Generalized LM presents with multifocal or osteolytic bony lesions, splenic involvement, as well as pleural and/or pericardial effusions. • Other terms for the generalized skeletal presentation include Gorham–Stout syndrome, disappearing bone disease, and phantom bone disease
- 60. DIAGNOSIS • Large or deep LMs are assessed by MRI to: (1) confirm the diagnosis; (2) Define the extent of the malformation; (3) plan treatment. • LM appears as either a macrocystic, microcystic or combined lesion with septations of variable thickness. • LM shows abnormally walled vascular spaces with eosinophilic, protein-rich fluid, and collections of lymphocytes. • Immunostaining with the lymphatic markers D2–40 and LYVE-1 are positive.
- 61. Management • LM is a benign lesion; intervention is not mandatory. • Small or asymptomatic lesions may be observed. • An infected LM often cannot be controlled with oral antibiotics and intravenous antimicrobial therapy • Intervention for LM is reserved for symptomatic lesions that cause pain, significant deformity, or threaten vital structures.
- 62. Sclerotherapy • Sclerotherapy is first-line management for large or problematic macrocystic/combined LM . • Sclerosants : doxycycline, sodium tetradecyl sulfate (STS), ethanol, bleomycin, and OK-432. • Doxycycline is 1st choice because it is effective (83% reduction in size) and safe (<5% risk of skin ulceration). • STS is second-line agent. • Ethanol is an effective sclerosant but has the highest complication rate. • Ethanol can injure nerves, and thus should not be used in proximity to important structures. • OK-432 is limited because it is not widely available.
- 63. Complication of sclerotherapy • Cutaneous ulceration (<5%). • Ethanol : CNS depression, pulmonary hypertension, hemolysis, thromboembolism, and arrhythmias. • Extravasation of the sclerosant into muscle can cause atrophy and contracture. • LM often re-expands over time.
- 64. Resection • Significant morbidity: major blood loss, iatrogenic injury, and deformity. • For example, resection of cervicofacial LM can injure the facial nerve (76%) or hypoglossal nerve (24%). • Excision is usually subtotal because LM involves multiple tissue planes and important structures; • recurrence is common (35–64%). • Resection is reserved for: • (1) symptomatic microcystic LM causing bleeding, infection, distortion of vital structures, or significant deformity; (2) symptomatic macrocystic/ combined LM that no longer can be managed with sclerotherapy because all macrocysts have been treated; (3) small, well-localized LM (microcystic or macrocystic) that may be completely excised.
- 66. Pathogenesis • Venous malformation (VM) results from an error in vascular morphogenesis. Lesions are composed of thin walled, dilated, sponge-like channels of variable size and mural thickness. • There is a normal-appearing endothelial lining; it is the smooth muscle architecture that is abnormal. Smooth muscle alpha-actin staining demonstrates decreased smooth muscle cells that are arranged in clumps rather than concentrically. • This mural abnormality probably accounts for the tendency of these malformations to gradually expand over time.
- 67. • Glomuvenous malformation (GVM) is the most common type. • GVM is an autosomal dominant condition with abnormal smooth muscle-like glomus cells along the ectatic veins. It is caused by a loss-of- function mutation in the glomulin gene. • Cutaneomucosal-venous malformation (CMVM) is rare. CMVM is an autosomal dominant condition caused by a gain-of-function mutation in the TIE2 receptor.
- 68. Clinical features • VMs are blue, soft, and compressible; calcified phleboliths often can be palpated. VM range from small, localized cutaneous lesions to diffuse malformations involving multiple tissue planes, vital structures, and internal organs. • VM is typically sporadic and solitary in 90% of patients.Sporadic VM is usually greater than 5 cm. • GVMs are typically multiple (70%), small (two-thirds <5 cm), and located in the skin and subcutaneous tissue; Lesions are more painful than typical VM. • Almost all lesions involve the skin, mucosa, or subcutaneous tissue; 50% also affect deeper structures.
- 69. • CMVM (Cutaneomucosal-venous malformation) are multifocal mucocutaneous lesions; they are less common than GVM. • Lesions are small (76% <5 cm), multiple (73%), and located on the head/neck (typically tongue or buccal mucosa) .
- 70. Blue rubber bleb nevus syndrome (BRBNS) • Blue rubber bleb nevus syndrome (BRBNS) is a rare condition characterized by multiple, small (<2 cm) VMs involving the skin, soft tissue, and gastrointestinal tract. • Morbidity is associated with gastrointestinal bleeding requiring chronic blood transfusions. • Diffuse phlebectasia of Bockenheimer is an old eponym to specify an extensive extremity VM involving skin, subcutaneous tissue, muscle, and bone. • Verrucous hemangioma (VH) is a low-flow vascular malformation that is clinically similar to a hyperkeratotic VM (“verrucous VM).
- 71. • Maffucci Syndrome denotes the coexistence of cutaneous venous malformations with bony exostoses and enchondromas.Osseous lesions appear first, most often in the hands, feet, long bones of the extremity, ribs, pelvis, and cranium. Recurrent fractures are common.
- 72. Complications of venous malformation • pain, swelling, and psychosocial issues. • Head and neck VM - mucosal bleeding or progressive distortion leading to airway or orbital compromise. • Extremity VM can Leg-length discrepancy, hypoplasia due to disuse atrophy, pathologic fracture, hemarthrosis, and degenerative arthritis. • Thrombosis and pulmonary embolism. • Gastrointestinal VM - bleeding and chronic anemia. • localized intravascular coagulopathy (LIC) and painful phlebothromboses.
- 73. Management • Custom-fitted compression garments to reduce blood stagnation and minimize expansion, phlebolith formation, and pain. • Prophylactic daily aspirin (81 mg) to prevent thrombosis. • Low molecular weight heparin (LMWH) is considered for patients with significant LIC who are at risk for DIC. • Long-term anticoagulation or a vena caval filter.
- 74. Sclerotherapy • First-line treatment is sclerotherapy, which is safer and more effective than resection. • Sclerotherapy is repeated until symptoms are alleviated or when injectable vascular spaces are no longer present. • VM usually re-expands after sclerotherapy. • The preferred sclerosants for VM are sodium tetradecyl sulfate (STS) and ethanol; STS is the most commonly used. Although ethanol is more effective than STS, it has a higher complication rate
- 75. Resection Resection is rarely primary treatment because: (1) the entire lesion is difficult to remove; (2) the risk of recurrence is high because hidden channels (3) the risk of blood loss and iatrogenic injury Resection should be considered for: (1) small well-localized lesions that can be completely removed, (2) persistent mass or deformity after completion of sclerotherapy
- 77. Pathogenesis • Arteriovenous malformation (AVM) is believed to result from an error of vascular development between the 4th and 6th weeks of gestation. • Halsted wrote that it results from failure of arteriovenous channels in the primitive retiform plexus to regress. • This may explain why AVM is 20 times more common in the central nervous system, where apoptosis is rare. • An absent capillary bed causes shunting of blood directly from the arterio-venous circulation, through a fistula or nidus (abnormal channels bridging the feeding artery to the draining veins).
- 78. • Capillary malformation-arteriovenous malformation (CM- AVM) is caused by a mutation in RASA1. • AVM may enlarge because of increased blood flow causing collateralization, dilatation of vessels (especially venous ectasia), and thickening of adjacent arteries and veins. • Latent arteriovenous shunts may open, stimulating hypertrophy of surrounding vessels from increased pressure. • Angiogenesis (growth of new blood vessels from pre-existing vasculature) and vasculogenesis (de novo formation of new vasculature) also may be involved in AVM expansion.
- 79. • Neovascularization may be a primary stimulus for AVM growth, it also could be a secondary event. For example, ischemia, a potent stimulator of angiogenesis, causes enlargement of AVM after proximal arterial ligation or trauma. • Increased blood flow due to arteriovenous shunting may promote angiogenesis; vascular endothelial growth factor (VEGF) production and endothelial proliferation are stimulated by increased blood flow. • Both males and females have a two-fold risk of progression in adolescence; increased circulating hormones during this period may promote AVM expansion.
- 80. Clinical features • The most common site of extracranial AVM is the head and neck, followed by the limbs, trunk, and viscera. • Although present at birth, AVM may not become evident until childhood. • Early lesions present as a pink-red cutaneous stain, without a palpable thrill or bruit. • Pain, ulceration, and bleeding. • Disfigurement, destruction of tissues, and obstruction of vital structures. • High pressure shunting of blood may lead to venous hemorrhage. • Arterial bleeding • Cardiac enlargement and result in high-output.
- 82. Diagnosis • History and physical examination. • Hand-held Doppler reveals fast-flow and excludes a slow- flow vascular anomaly. • US with color Doppler examination. • MRI also is necessary to: (1) confirm the diagnosis; (2) determine the extent of the lesion; (3) plan treatment. • Angiography - prior to embolization or when resection is planned. • CT may be indicated if the AVM involves bone.
- 83. Management • Because AVM is often diffuse, involving multiple tissue planes and important structures, cure is rare. The goal of treatment usually is to control the malformation. • Intervention is focused on alleviating symptoms (i.e., bleeding, pain, ulceration), preserving vital functions (i.e., vision, mastication), and improving deformity. • Management options include embolization, resection, or a combination. • Resection offers the best chance for long-term control, but the re-expansion rate is high. • Almost all AVMs will re-expand after embolization. • Embolization is used to reduce blood loss during resection or sometimes for palliation of unresectable lesions.
- 84. • Intervention is determined by: (1) the size and location of the AVM; (2) the age of the patient; (3) Schobinger stage. • Large, asymptomatic AVM located in an anatomically sensitive area is best observed; especially in a young child who is not psychologically ready for major resection and reconstruction.
- 85. Embolization • Embolization is the delivery of an inert substance, through a catheter into the AVM nidus to occlude blood flow and/or fill a vascular space. • Embolization is used either as a preoperative adjunct to resection or as monotherapy for lesions not amenable to extirpation. • Stage I AVM has a lower recurrence rate than higher-staged lesions. • Most recurrences occur within the first year after embolization, and 98% re-expand within 5 years. • Preoperative embolization also reduces blood loss during extirpation, but not the extent of resection. • Substances used for embolization are either liquid (n-butyl cyanoacrylate (n-BCA), Onyx) or solid (polyvinyl alcohol particles (PVA), coils).
- 86. Resection • Resection of AVM has a lower recurrence rate than embolization alone; it is considered for a well-localized lesion or to correct deformity (i.e., bleeding or ulcerated areas, labial hypertrophy). • Wide extirpation and reconstruction of large, diffuse AVM should be exercised with caution because: (1) cure is rare and the recurrence rate is high; (2) the resulting deformity is often worse than the appearance of the malformation; (3) resection is associated with significant blood loss, iatrogenic injury, and morbidity. • Multiple embolizations, spaced 6 weeks apart, may be required prior to resection. Excision should be done 24–72 h after embolization, before recanalization restores blood flow to the lesion.
- 87. Capillary malformation-arteriovenous malformation • Capillary malformation-arteriovenous malformation (CM- AVM) is an autosomal dominant condition caused by a loss-of- function mutation in the RASA1 gene. • The prevalence is 1 in 100 000 Caucasians. • Patients have atypical capillary malformations (CMs) that are small, multifocal, round, pinkish-red, and often surrounded by a pale halo.
- 88. Eponymous vascular anomalies with overgrowth
- 89. Sturge–Weber syndrome • Sturge–Weber syndrome (SWS) is a sporadic neurocutaneous disorder estimated to occur in 1 in 50 000 live births. • The three cardinal features are Capillary malformation (CM) in the upper trigeminal neural distribution, ocular abnormalities (glaucoma, choroidal vascular anomalies), leptomeningeal vascular malformation. • Patients also commonly have soft tissue and/or bony overgrowth (60–83%); the frequency is similar to that for glaucoma (65–77%) and for neurological sequelae (87–93%).
- 90. • Patients with an upper facial CM, a diagnosis of Sturge–Weber syndrome should be considered on initial presentation. • The capillary stain can be in the ophthalmic (V1), extend into the maxillary (V2), or involve all three trigeminal dermatomes. • Patients with maxillary or mandibular involvement alone are at low risk for SWS. • The leptomeningeal anomalies can be capillary, venous, or arteriovenous malformations. • Small foci may be silent, but extensive pial vascular lesions can cause refractory seizures, contralateral hemiplegia, and delayed motor and cognitive development.
- 91. Klippel–Trénaunay syndrome • Klippel–Trénaunay syndrome (KTS) denotes a slow flow, capillary- lymphatic-venous malformation (CLVM) of an extremity in association with soft tissue and/or skeletal overgrowth . • It is often incorrectly called “Klippel–Trénaunay–Weber”, invoking Parkes Weber syndrome, a fast-flow vascular malformation. • Slightly enlarged extremity with a capillary stain to a grotesquely enlarged limb with malformed digits. • Affects the lower extremity in 95% of patients, the upper extremity in 5% of patients, and least commonly the trunk. • Sometimes the contralateral foot or hand is enlarged, often with a macrodactylous component and frequently in the absence of a capillary stain. • In 10% of patients with KTS, the involved limb is hypoplastic. • Pelvic involvement is common with CLVM of the lower extremity. • The venous component of CLVM manifests as abnormal drainage of
- 92. Parkes Weber syndrome • Parkes Weber syndrome (PWS) is a diffuse AVM in an overgrown extremity with an overlying CM . • PWS involves the lower extremity approximately twice as often as the upper extremity; patients have microshunting in muscle. • The malformation is evident at birth with symmetric enlargement and pink staining of the involved limb. • The cutaneous stain tends to be confluent rather than patchy and is typically warmer than a banal capillary malformation. • The diagnosis is confirmed by the detection of a bruit or thrill. • MRI is obtained to evaluate the extent of the malformation. • Overgrowth in an affected extremity is subcutaneous, muscular, and bony with diffuse microfistulas. • The enlarged limb muscles and bones exhibit an abnormal signal and enhancement. • Angiography demonstrates discrete arteriovenous shunts.
- 93. PTEN hamartoma-tumor syndrome (Bannayan– Riley–Ruvalcaba syndrome) • Patients with PTEN mutations, a tumor suppressor gene, have PTEN hamartoma-tumor syndrome (PHTS). • This autosomal dominant condition is also referred to as Cowden syndrome or Bannayan–Riley– Ruvalcaba syndrome. • Males and females are equally affected, and approximately one-half (54%) of patients have a unique fast-flow vascular anomaly with arteriovenous shunting, referred to as a PTEN-associated vascular anomaly. • Patients may have multiple lesions (57%), and 85% are intramuscular. • PHTS is associated with mental retardation/autism (19%), thyroid lesions (31%), or gastrointestinal polyps (30%). • Biopsy may aid the diagnosis of a PTEN fast-flow lesion
- 94. CLOVES syndrome • Congenital Lipomatosis Overgrowth, Vascular malformations, Epidermal nevi, and Scoliosis (CLOVES) represents a newly described overgrowth syndrome. • Many of these patients previously were thought to have “Proteus syndrome”. • Unlike Proteus syndrome, patients with CLOVES do not have skeletal involvement and the soft-tissue component is not progressive. • All patients have a truncal lipomatous mass, a slowflow vascular malformation (most commonly a CM overlying the lipomatous mass), and hand/foot anomalies (increased width, macrodactyly, first web-space sandal gap). • Patients also may have AVM (28%), neurological impairment (50%), or scoliosis (33%). • Treatment for the lipomatous lesions is resection, but the recurrence rate is high.
