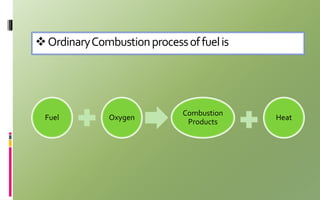
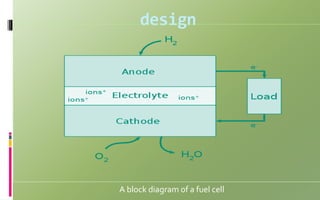
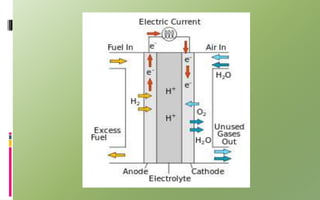
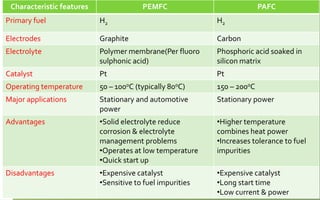
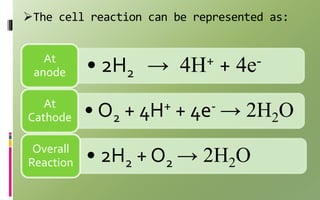
Fuel cells convert chemical energy directly into electrical energy through electrochemical reactions. They consist of an anode, cathode, and electrolyte. In the process, hydrogen atoms are split into protons and electrons at the anode; protons pass through the electrolyte while electrons flow through an external circuit, generating electricity. At the cathode, protons and electrons combine with oxygen to form water. Fuel cells are more efficient and less polluting than combustion engines. Two major types are phosphoric acid fuel cells (PAFC) and polymer electrolyte membrane fuel cells (PEMFC), which differ in their electrolytes, operating temperatures, and applications. Fuel cells are used to power vehicles, buildings, and portable devices.