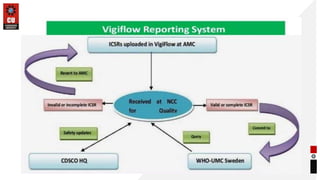
This document discusses methods and tools for adverse drug reaction (ADR) reporting. It defines ADR reporting and its importance. Anyone can report ADRs by filling out a standard form available online or offline and submitting it to their nearest monitoring center. Common tools for ADR reporting include Argus Oracle and Aris G software used by drug manufacturers, and Vigiflow and Vigibase databases maintained by the WHO. Healthcare professionals and consumers can report any suspected ADRs using spontaneous reporting or other methods to national pharmacovigilance centers.