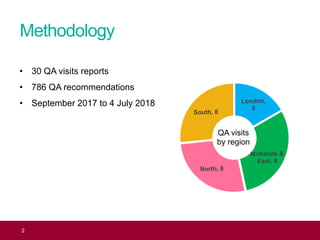
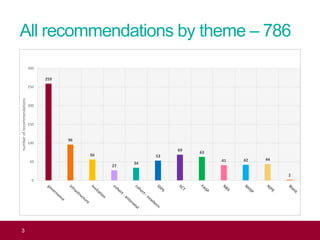
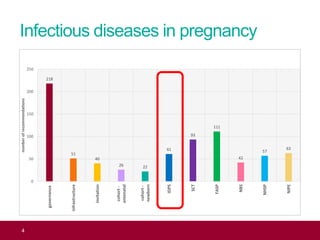
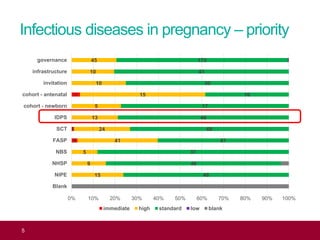
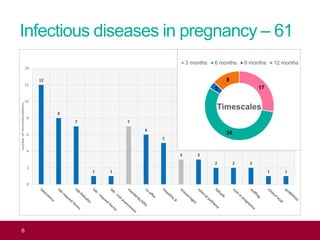
The QA advisor summarizes findings from 30 QA visits reports between September 2017 and July 2018. A total of 786 QA recommendations were made across various themes. The top theme was infectious diseases in pregnancy, receiving 218 recommendations. Of these, 61 recommendations were specific to the laboratory, focusing on issues like properly identifying and tracking antenatal samples, notifying screening results, and ensuring UKAS accreditation. Generic recommendations that appeared across themes included formalizing governance, managing incidents, and meeting standards for staff training and turnaround times.