Clinical Trial Management
•Download as PPTX, PDF•
0 likes•22 views
Novum manages clinical endpoint studies that require large patient populations in multiple disease states, treated in an outpatient setting. With a nationwide network of more than 2,000 investigator sites, we ensure that all selected sites meet the requirements of each study and Novum’s high standards.
Report
Share
Report
Share
Recommended
Digital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Virtual, & Decentralized Clinical TrialsDigital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Vi...

Digital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Vi...SC CTSI at USC and CHLA
More Related Content
Similar to Clinical Trial Management
Digital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Virtual, & Decentralized Clinical TrialsDigital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Vi...

Digital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Vi...SC CTSI at USC and CHLA
Similar to Clinical Trial Management (20)
Clinical Research Associate Resume. Why Choose Bes

Clinical Research Associate Resume. Why Choose Bes
Leverage Big Data Analytics to Enhance Clinical Trials from Planning to Execu...

Leverage Big Data Analytics to Enhance Clinical Trials from Planning to Execu...
Digital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Vi...

Digital Scholar Webinar: Breaking Down (Brick) Walls: Switching to Remote, Vi...
Designing of clinical study protocol rumana hameed

Designing of clinical study protocol rumana hameed
Roles and Responsibilities of sponsor, CRO, and investigator 

Roles and Responsibilities of sponsor, CRO, and investigator
Recently uploaded
Young & Hot ℂall Girls Salem 8250077686 WhatsApp Number Best Rates of Surat ℂall Girl Serviℂes Available 24x7x365 Young & Hot ℂall Girls Salem 8250077686 WhatsApp Number Best Rates of Surat ℂ...

Young & Hot ℂall Girls Salem 8250077686 WhatsApp Number Best Rates of Surat ℂ...Call Girls in Nagpur High Profile Call Girls
Recently uploaded (20)
Connective Tissue II - Dr Muhammad Ali Rabbani - Medicose Academics

Connective Tissue II - Dr Muhammad Ali Rabbani - Medicose Academics
^In Pietermaritzburg Hager Werken Embalming +27789155305 Compound Powder in ...

^In Pietermaritzburg Hager Werken Embalming +27789155305 Compound Powder in ...
Bhimrad + ℂall Girls Serviℂe Surat (Adult Only) 8849756361 Esℂort Serviℂe 24x...

Bhimrad + ℂall Girls Serviℂe Surat (Adult Only) 8849756361 Esℂort Serviℂe 24x...
Kamrej + ℂall Girls Serviℂe Surat (Adult Only) 8849756361 Esℂort Serviℂe 24x7...

Kamrej + ℂall Girls Serviℂe Surat (Adult Only) 8849756361 Esℂort Serviℂe 24x7...
Mgr university bsc nursing adult health previous question paper with answers

Mgr university bsc nursing adult health previous question paper with answers
TEST BANK For Huether and McCance's Understanding Pathophysiology, Canadian 2...

TEST BANK For Huether and McCance's Understanding Pathophysiology, Canadian 2...
Histology of Epithelium - Dr Muhammad Ali Rabbani - Medicose Academics

Histology of Epithelium - Dr Muhammad Ali Rabbani - Medicose Academics
Treatment Choices for Slip Disc at Gokuldas Hospital

Treatment Choices for Slip Disc at Gokuldas Hospital
Tips and tricks to pass the cardiovascular station for PACES exam

Tips and tricks to pass the cardiovascular station for PACES exam
Gross Anatomy and Histology of Tongue by Dr. Rabia Inam Gandapore.pptx

Gross Anatomy and Histology of Tongue by Dr. Rabia Inam Gandapore.pptx
Young & Hot ℂall Girls Salem 8250077686 WhatsApp Number Best Rates of Surat ℂ...

Young & Hot ℂall Girls Salem 8250077686 WhatsApp Number Best Rates of Surat ℂ...
Unveiling Alcohol Withdrawal Syndrome: exploring it's hidden depths

Unveiling Alcohol Withdrawal Syndrome: exploring it's hidden depths
ANAPHYLAXIS BY DR.SOHAN BISWAS,MBBS,DNB(INTERNAL MEDICINE) RESIDENT.pptx

ANAPHYLAXIS BY DR.SOHAN BISWAS,MBBS,DNB(INTERNAL MEDICINE) RESIDENT.pptx
How to buy 5cladba precursor raw 5cl-adb-a raw material

How to buy 5cladba precursor raw 5cl-adb-a raw material
Clinical Trial Management
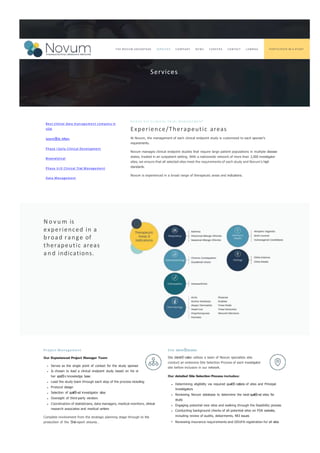
- 1. P H A S E II-IV C L I N I C A L T R I A L M A N A G E M E N T Experience/Therapeutic areas At Novum, the management of each clinical endpoint study is customized to each sponsor’s requirements. Novum manages clinical endpoint studies that require large patient populations in multiple disease states, treated in an outpatient setting. With a nationwide network of more than 2,000 investigator sites, we ensure that all selected sites meet the requirements of each study and Novum’s high standards. Novum is experienced in a broad range of therapeutic areas and indications. Best clinical data m a n a g e men t company in USA Scienti몭 c Affairs Phase I Early Clinical Development Bioanalytical Phase II-IV Clinical Trial Management Data Management N o v u m is experienced in a broad range of therapeutic areas an d indications. Services Site Identi몭 cation Site Identi몭 cation utilizes a team of Novum specialists who conduct an extensive Site Selection Process of each investigator site before inclusion in our network. Our detailed Site Selection Process includes: Project Management Our Experienced Project Manager Team Serves as the single point of contact for the study sponsor Is chosen to lead a clinical endpoint study based on his or her speci몭 c knowledge base Lead the study team through each step of the process including Protocol design Selection of quali몭 ed investigator sites Oversight of third-party vendors Coordination of statisticians, data managers, medical monitors, clinical research associates and medical writers Complete involvement from the strategic planning stage through to the production of the 몭n a l report ensures… Determining eligibility via required quali몭 cations of sites and Principal Investigators Reviewing Novum database to determine the best-quali몭 ed sites for study Engaging potential new sites and walking through the feasibility process Conducting background checks of all potential sites on FDA website, including review of audits, debarments, 483 issues Reviewing insurance requirements and GDUFA registration for all sites T H E N O V U M ADVANTAG E S E R V I C E S C O M P A N Y N E W S C A R E E R S CONTACT L A M B D A PARTI CI PATE IN A STUDY
- 2. 225 W. Station Square Drive Suite 200 Pittsburgh, PA 15219 © 2023, Novum. All rights reserved. | PRIVACY POLICY Site by Monitoring Our experienced Project Managers and our skilled Clinical Research Associates work together as a team to e몭 ciently manage site activity. Novum Monitoring Services include: Proper execution The highest-quality deliverables Timely completion of each clinical endpoint study Conducting on-site Pre-Study Site Selection and Site Initiation review Monitoring on-site Source-to-CRF veri몭 cation Reviewing documents to ensure regulatory compliance Reviewing study drug for appropriate handling Resolving Data Queries Reporting 몭 ndings to the Project Manager Maintaining Trial Master File Our team concept allows our Project Managers to manage a study from beginning to end and maintain real-time knowledge of the activities occurring at all sites throughout the clinical endpoint study, while allowing our CRAs to focus on the speci몭 c needs of individual sites to ensure the successful completion of each study. Upon completion of a study, our Site Selection Department gathers feedback from the investigator sites, assessments from Novum CRAs assigned to the study and historical data regarding each site to determine future use of the site. Quali몭 ed investigator sites A network of more than 2,000 investigator sites in the USA, Puerto Rico and Latin American countries Background checks and inspections of each site’s recruitment capabilities, facilities and regulatory history Validation of each site’s experience to ensure it meets the requirements of the studies Ready to get started? Find out how Novum can make the di몭 erence on your next clinical trial. R E Q U E S T A Q U O T E