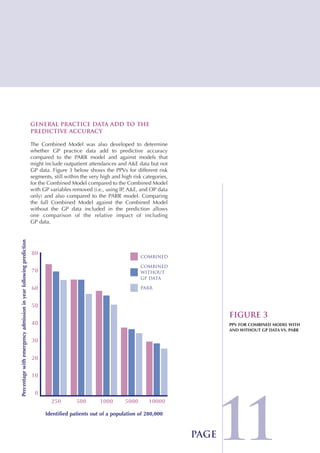
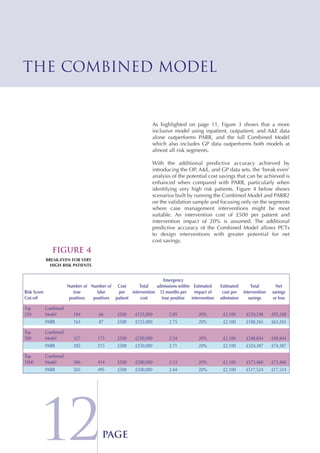
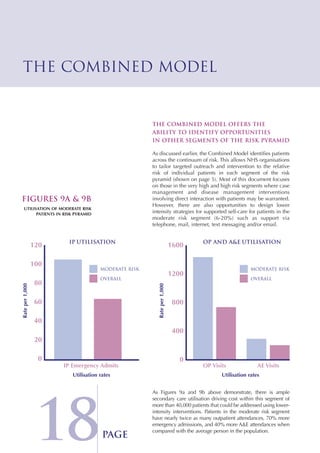
The Combined Predictive Model final report describes a new predictive model that was developed using data from multiple sources, including inpatient, outpatient, emergency room, and primary care records. This model segments patients into risk levels and can help the NHS target interventions appropriately based on patients' predicted risk levels. It improves on previous models by identifying a broader range of at-risk patients and allowing for stratified approaches along the continuum of care. The report concludes the model can help design long term condition programs that match intervention intensity to patients' needs.