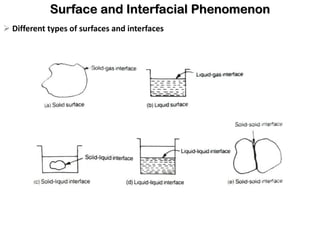
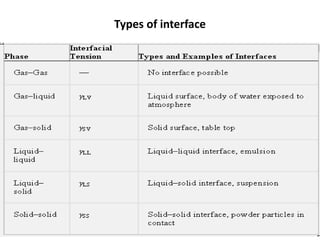
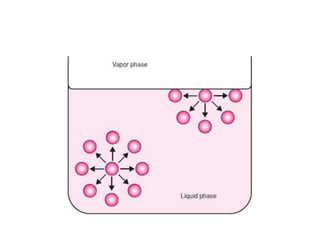
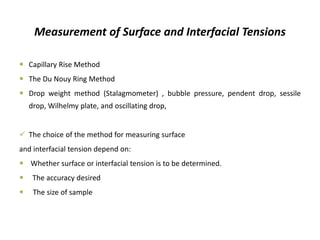
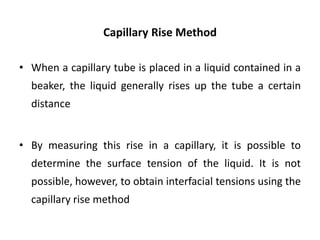
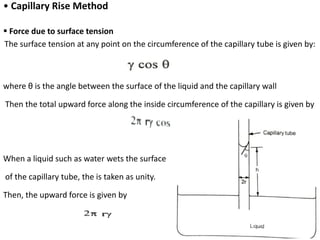
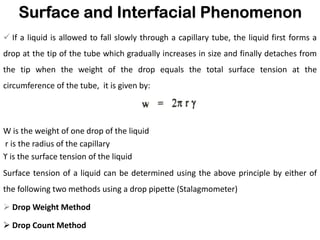
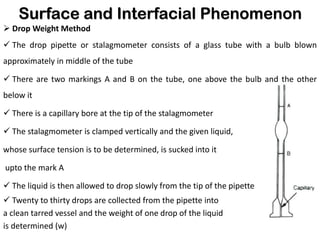
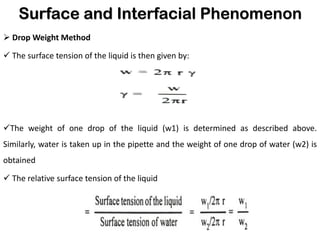
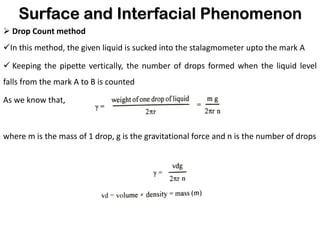
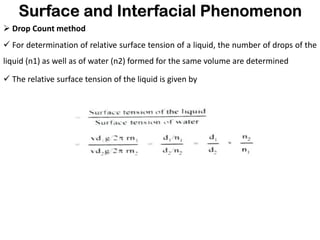
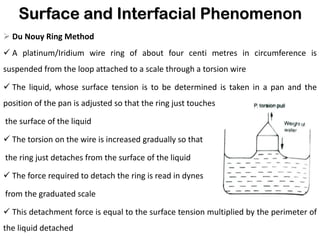
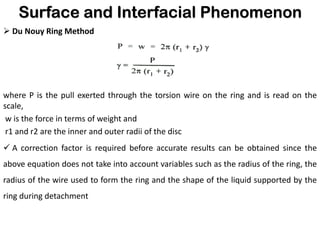
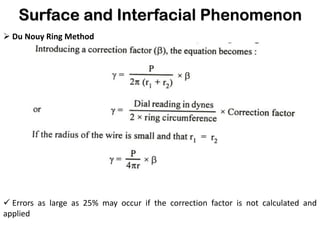
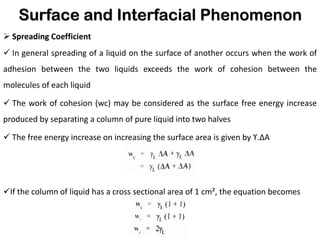
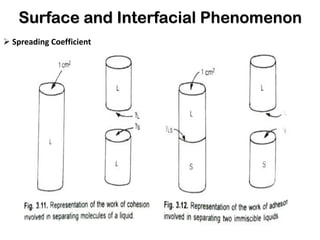
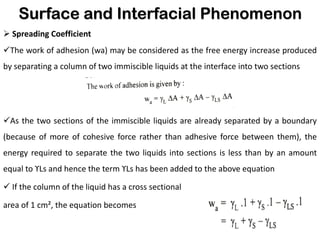
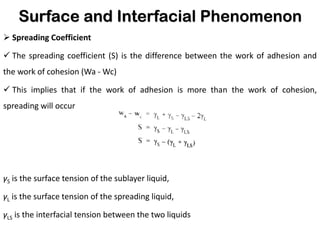
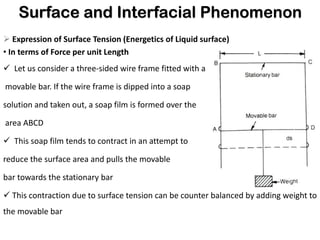
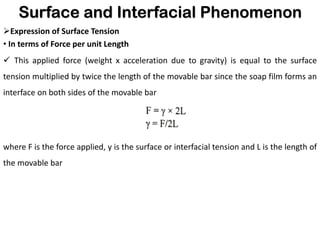
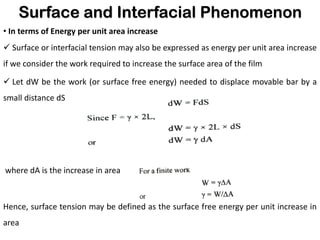
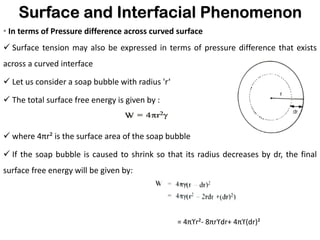
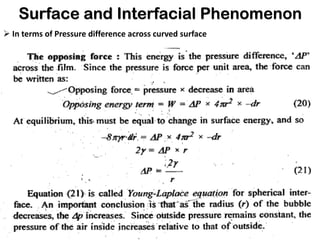
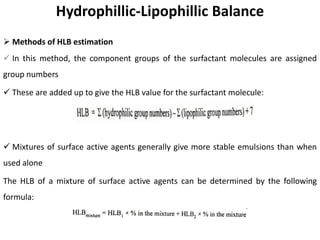
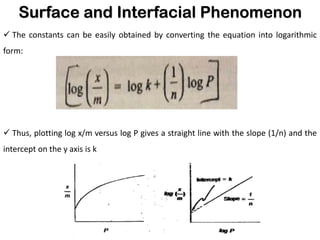
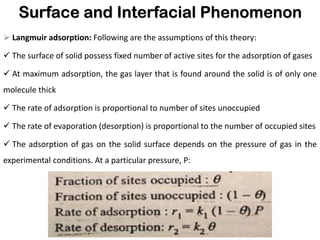
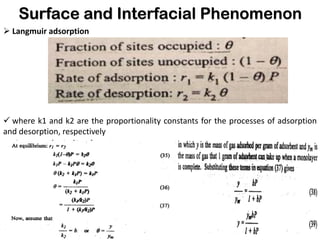
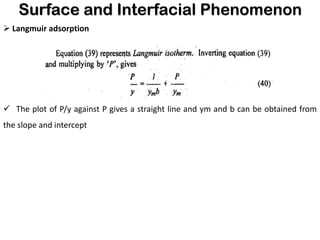
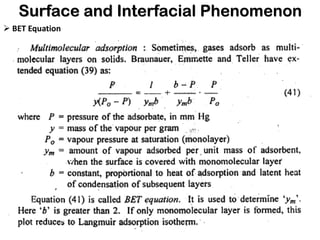
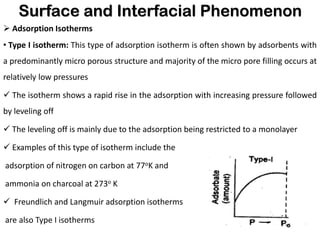
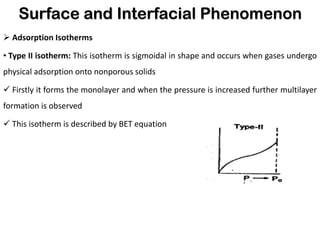
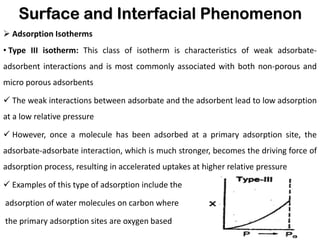
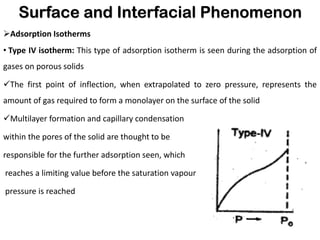
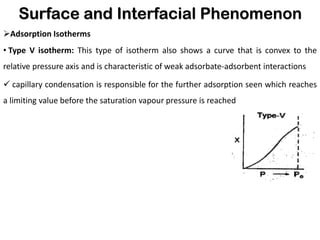
This document discusses surface and interfacial phenomena. It defines interfaces and surfaces, and describes different types of interfaces including liquid interfaces. It explains concepts such as surface tension, interfacial tension, and surface free energy. Methods for measuring surface and interfacial tensions like the capillary rise method, Du Nouy ring method, and drop weight method are summarized. The document also discusses spreading coefficients and adsorption at liquid interfaces.