Biomaterials in the sustainability of regenerative medicine
- 1. Biomaterials in Sustainability of Regenerative Medicine Michael N. Helmus, Ph.D., Consultant Medical Devices, Biomaterials, Drug Delivery, and Nanotechnology mnhelmus@msn.com
- 2. Biomaterials in Sustainability of Tissue Engineering Biomaterials for Regenerative Medicine Scaffolds Biomaterials - Categories - Biocompatibility - Regenerative Medicine applications Identification of Drivers for New Technology - Leverage Potential Emergent/Disruptive Technology Enhanced biocompatibility for Regenerative Medicine is a Disruptive Medical Technology
- 3. 21st Century
- 5. HYBRID ARTIFICIAL ORGANS Galletti P. M. Chairman; Hori, M.; Sharp, D. W.; Stanley, J. C.ASAIO Journal: April 1982 - Volume 28 - Issue 1 - ppg 639-646
- 8. – FDA Web Page - Center For Devices: MDR’s, Press Releases http://www.fda.gov/MedicalDevices/default.htm – 510K Home page http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClear ances/510kClearances/default.htm – International Standards Organization (ISO): http://www.iso.org/iso/iso_catalogue/catalogue_ics/catalogue_ics_browse.htm?ICS1=11Standa rds – FDA recognized consensus standards are listed at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfstandards/search.cfm •Medical devices – AdvaMed http://www.advamed.org/MemberPortal/ – Email updates: http://www.smartbrief.com/news/ADVAMED/index.jsp?categoryid=7B651A9C- 543B-43A9-909D-CC5F80F69335 – Medical Device and Diagnostic Industry: http://www.mddionline.com/ •Web sources for Biomaterials, Guidelines and Standards
- 9. •Materials information – Society for Biomaterials: http://www.biomaterials.org/ – Biomaterials.net: http://www.biomat.net/ – ASM International (subscription required): http://products.asminternational.org/meddev/index.aspx – MatWeb (General materials with search term medical grade): http://www.matweb.com/index.aspx – History of Biomaterials http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552- 4965/homepage/VirtualIssuesPage.html – Advances in Biomaterials http://www.intechopen.com/books/advances-in-biomaterials-science-and- biomedical-applications •Clinical information – General: http://www.medscape.com – NIH Reporter http://projectreporter.nih.gov/reporter.cfm
- 10. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com STRUCTURAL MATERIALS SURFACE MATERIALS: BIOLOGIC INTERACTIONS AND LUBRICITY CONTROLLED DRUG DELIVERY MATERIALS METALS ENGINEERING PLASTICS PLASTICS ELASTOMERS CERAMICS BIOACTIVE CERAMICS BIOACTIVE COATINGS BIOLOGICS BIODERIVED MACROMOLECULES HYDROPHILIC COATINGS HIGH STRENGTH MODERATE STRENGTH HIGH PERMEABILITY SURFACE COATINGS SPECTRUM OF MATERIALS AND PROPERTIES Bioactivity COMPOSITES AEROSPACE DEFENSE ORTHOPEDIC DENTAL RESEARCH PHARMACEUTICAL AND BIOTECH Plastics & Textiles Industry
- 11. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com STRUCTURAL MATERIALS SURFACE MATERIALS: BIOLOGIC INTERACTIONS AND LUBRICITY CONTROLLED DRUG DELIVERY MATERIALS METALS ENGINEERING PLASTICS PLASTICS ELASTOMERS CERAMICS BIOACTIVE CERAMICS BIOACTIVE COATINGS BIOLOGICS BIODERIVED MACROMOLECULES HYDROPHILIC COATINGS HIGH STRENGTH MODERATE STRENGTH HIGH PERMEABILITY SURFACE COATINGS SPECTRUM OF MATERIALS AND PROPERTIES Bioactivity COMPOSITES AEROSPACE DEFENSE ORTHOPEDIC DENTAL RESEARCH PHARMACEUTICAL AND BIOTECH Plastics & Textiles IndustryMEMS Nano technology Self Assembled Molecules Biomimetics Tissue Engineering/Regenerative Medicine 3D Printing
- 12. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Materials Selection Guide Identify: • Predicate Devices • Corporate/Institutional Predicate Devices, Testing, and Regulatory Approvals (510(k)s, PMA’s, and NDA’s) • Corporate/Institutional Guidelines, Procedures and Protocols • FDA Guidelines, CEN Guidelines, and Standards (ASTM, ANSI, ISO) • Corporate/Institutional R&D Reports • Materials, Uses, Properties, ASTM and ISO Standards Develop an Approach for Selection and Testing
- 13. Materials used in medical devices, particularly in those applications in which the device either contacts or is temporarily inserted or permanently implanted in the body, are typically described as biomaterials and have unique design requirements. The National Institute of Health Consensus Development Conference of November 1982 defined a biomaterial as “any substance (other than a drug) or combination of substances, synthetic or natural in origin, which can be used for any period of time, as a whole or as a part of a system which treats, augments, or replaces any tissue, organ, or function of the body” http://tpx.sagepub.com/content/36/1/70.full.pdf Biomaterials
- 14. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com • Scaffolding for tissue regeneration and replacement • hybrid artificial organs and bioegineered tissues. Acceptable scaffolding materials • Biocompatible • allow cellular interactions that result in tissue that mimics • naturally occurring material • biochemical • biomechanical Scaffolds are Biomaterials for Regenerative Medicine Applications
- 15. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com • Tissue Engineered devices have a design requirement that meet the physical properties of the device/replaced organ •Acute •Chronic •Non-degradable •Biodegradable
- 16. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com •Remodeled biologic •Homograft, allograft, xenograft •Calicification mitigation •Decellularization •Growth factors •Bioactive agents •incorproated into the substrates to encourage the proper cell •Reconstituted macromolecules •Recellularization •bioreactor •in situ
- 17. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com SELECTED HISTORY • Noshiki 1996 presaged this work when he used bone marrow cells to recellularize traditional polyester prostheses. •Work by Van Kampen 1979 at Case showed that mononuclear cells formed islands of endothelium on the coagulum of vascular implants, also seen in work of M. Helmus • Cell adhesion proteins like fibronectin and RGD peptides help in capturing circulating endothelial precursor cells.
- 18. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com • Immobilized Fibronectin and Type IV Collagen on ePTFE and porous polyurethane by BioMetrics Systems( now Surmodics ) demonstrated enhanced endothelialization (Clapper). • RGD peptides (Peptite 2000 from Telios Pharmaceuticals) on PET and PTFE fabric in canine carotid and femoral patches demonstrated enhanced reendothelialization • Orbus has developed an immobilized antibody to capture circulating endothelial progenitor cells (EPCs) in order to enhance this process. • BioSet has growth factor mimetics which may have a role in enhancing recellularization.
- 19. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com (Van Kampen 1979)
- 20. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Reendothelialized Fibrin Coated ePTFE Vascular Graft Tricuspid SIS Heart Valve Mesenchymal Mesh for Hernia Repair Biomaterial Scaffolds for Medtech Tissue Engineered Bone Segments Porous tantalum Mesenchymal Stem Cell Spinal Fusion Control of Wound Healing RGD Peptides Scaffolds for Tissue engineering Bioactive and Bioresorbable Fabrics Type 3 collagen acellular vascular graft Acelllular Uterine Sling -human dermis
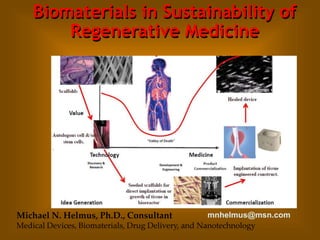
- 21. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Implantation of tissue engineered construct. Tissue Engineered Devices Biopsy/tissue sample for autologous cell &/or isolation of stem cells. Culturing of cells to expand if needed. Scaffolds: Decellularized tissue, Polymer, Biodegradables, Bioderived, eg collagen Seeded scaffolds for direct implantation or growth of tissue in bioreactor Healed device
- 22. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Small Molecules Proteins/Growth Factors Gene Transfection Remodeled Organ In Situ Healing Injectables to recruit bmc’s/tissue stem cells to Regenerate in situ.
- 23. •Materials for FDA-approved implantable devices •ASM International/Granta •M. N. Helmus, Ph.D. •Chair Medical Materials Database Committee •http://mio.asminternational.org/mmd/ New Tools for Medical Device Design
- 24. Materials for Medical Devices Biological Materials Bioprosthetic, Autologous, Allografts, Xenografts, ECM, Polysaccharides Carbonaceous Materials Pyrolytic, Graphitic, Graphene, Nanotube Ceramics Metals and Alloys Cobalt Base, Nitinol, Precious, Refractory Metals, Stainless Steels, Titanium Base Polymers/Plastics and Textiles Elastomers, Thermoplastics, Hydrogels, Engineering
- 29. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 30. The Research Portfolio Online Reporting site is a tool to search the NIH database of projects http://projectreporter.nih.gov This unique tool allows searching by fiscal year, NIH Center and Spending Category. A copy of the full search page is shown below. NIH Reporter Search NIH research projects
- 31. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com 3-D Printing: Unique fabrication method for biomaterials and devices Polymers, macromolecules, metals, ceramics, cells Scaffolds Textured and Porous surfaces Cellular tissue engineered structures 16 APRIL 2015 | VOL 520 | NATURE | 273
- 32. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com 16 APRIL 2015 | VOL 520 | NATURE | 273
- 33. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com At the Inside 3D Printing conference this week in New York, researchers from academia and industry are gathering to discuss the growing interest in using three- dimensional (3D) printing to make replacement body parts. Although surgeons are already using 3D-printed metal and plastic implants to replace bones, researchers are looking ahead to printing organs using cells as 'ink'. The structures shown here were all 3D printed at Wake Forest Baptist Medical Center in Winston-Salem, North Carolina, and include a rudimentary proto-kidney (top left), complete with living cells 16 APRIL 2015 | VOL 520 | NATURE | 273
- 34. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Artificial Organs May Finally Get a Blood Supply Artificial tissue has always lacked a key ingredient: blood vessels. A new 3-D printing technique seems poised to change that. •By Susan Young Rojahn on March 6, 2014 Why It Matters Thousands of people die each year waiting for donor organs. http://www.technologyreview.com/news/525161/artificial-organs-may-finally-get-a- blood-supply/
- 35. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Living layers: Harvard researchers demonstrate their method for creating vascularized tissue constructs by printing cell-laden inks in a layered zig-zag pattern. In what may be a critical breakthrough for creating artificial organs, Harvard researchers say they have created tissue interlaced with blood vessels. Using a custom-built four-head 3-D printer and a “disappearing” ink, materials scientist Jennifer Lewis and her team created a patch of tissue containing skin cells and biological structural material interwoven with blood-vessel-like structures. Reported by the team in Advanced Materials, the tissue is the first made through 3-D printing to include potentially functional blood vessels embedded among multiple, patterned cell types.
- 36. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com BIOMATERIALS Material Applications Synthetic Plastics, Engineering Plastics and Textiles Acrylics Housing materials for extracorporeal devices, such as blood pumps and oxygenators Ethylene vinyl acetate Wound dressings, drug delivery Epoxies Potting compound, fiber composites Fluorocarbons Vascular grafts, catheters, catheter components Hydrogels Coatings, drug delivery, contact lenses, sealants, embolics Polyacetal Heart valve components, catheter and structural components Poly(amides) Catheters and components, wound dressings, angioplasty balloons Poly(amide) elastomers Catheters, wound dressings, angioplasty balloons Poly(carbonates) Housing materials for extracorporeal devices Poly(esters) Angioplasty balloons, films and structural components Poly(ester) fibers Textile vascular grafts, fabrics Poly(ester) elastomers Catheters, angioplasty balloons Poly(etherketones) Structural components, fiber composites, orthopedic devices Poly(imides) Housing materials for extracorporeal devices Poly(methylpentene) Housing materials for extracorporeal devices Poly(olefins) Sutures, angioplasty balloons, catheters
- 37. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Expanded Polypropylene Membrane Hemodialysis Membranes, Controlled Drug delivery Poly(olefin) elastomers Tubing, artificial heart bladder, catheters, spinal discs High crystallinity poly(olefin) films Angioplasty balloons Poly(sulfones) Structural components, orthopedic devices, fiber composites Poly(urethanes) Catheters, artificial heart, wound dressings Poly(vinyl chloride) Tubing, blood Polyvinylidene fluoride Tubing, piezoelectric material Silicones Heart valve poppets, wound dressings, finger joints, reconstructive surgery Ultra-high molecular weight polyethylene Acetabular cup, high strength textiles
- 38. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Membranes to control diffusion - oxygenators, batteries Bioreactor components, Cell culture Scaffold Celgard 20- 80 nm pores, from the mid 1960’s
- 39. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 40. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 41. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Medtech Strategist Nov. 2014
- 42. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com UPDATED: J&J goes all in with ViaCyte, hands over BetaLogics assets in hunt for diabetes cure Feb 4, 2016
- 43. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Biocompatibility Issues of Biomaterials Synthetic Plastics, Engineering Plastics, Textiles, and Hydrogels Extractables Hypersensitivity reactions (e.g. latex materials) 2 part systems and cytotoxic residuals Lipid uptake Hydrolytic stability Biostability Biodegradation by-products Calcification Sterilization residuals Fatigue and wear particulates Protein adsorption: hydrophilic, hydrogel and hydrophobic
- 44. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Hydrogels Hydrocolloids Hydroxyethyl-methacrylate Ionic Acrylics – eg Acrylic acid based polymers Poly(acrylamide) Poly(ethylene oxide) Polysaccharides – eg Chitosans Hyaluronic acid Poly(vinlyalcohol) Poly(vinyl-pyrrolidone) Protein based – eg Gelatin, Albumin Extractables Hypersensitivity reactions Lipid uptake Hydrolytic stability Biostability Biodegradation by-products Sterilization residuals Calcification Blood Element Consumption Low protein adsorption Poor tissue adherence Fatigue and wear particulates
- 45. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Bioresorbables Poly(amino acids) Controlled release, cell adhesion peptides, scaffolds for tissue engineering Poly(anhydrides) Wound dressings, scaffolds Poly(caprolactones) Controlled release, sutures, scaffolds Poly(lactic/glycolic acid) copolymers Sutures, bone plates, controlled release, scaffolds Poly(lactide- caprolactone) Sutures, controlled release, bone plates, scaffolds Poly(lactic acid co lysine) Anti-adhesion spray for wounds, controlled release Poly(hydroxybutyrates ) Scaffolds for recellularization Poly(orthoesters) Controlled release, bone plates, scaffolds Poly(ester) elastomers Controlled release, bone plates Poly(dioxanone) Wound dressings, tympanic implants, sutures Poly(dimethyl trimethylene carbonate co- tirmethylene carbonate) Sutures Poly(phosphazenes) Controlled release Collagen Controlled release, scaffolds, sutures Low-density hydroxyapatite Coatings, soft tissue reconstruction, scaffold for recellularization bone implants, reconstructive surgery
- 46. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Biodegradables Rate of biodegradation Surface vs. bulk Particulates Biodegradation by-products Biodeposition Tissue partitioning and excretion Effect of infection (acidic pH) or hematoma (basic pH) on degradation rates Fatigue and wear particulates
- 47. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Tissue Engineering of Vascular Prosthetic Grafts by P. P. Zilla, Howard P. Greisler (Eds)
- 48. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 49. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Biologically Derived Materials Bovine arteries and veins Vascular grafts Bovine pericardium Pericardial substitute, heart valves Human umbilical vein Vascular grafts Human dura-mater Dura-mater replacement, reconstructive surgery Porcine heart valve Heart valves Bovine ligaments Ligaments Bovine tendons Tendons Decellularized tissues: SIS, Bladder Scaffolds for recellularization in vitro or in situ Decalcified bovine bone Bone implants Cross-linked bovine bone Bone implants
- 50. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com BBiioollooggiiccaallllyy DDeerriivveedd MMaatteerriiaallss:: AArrtteerriieess,, vvaallvveess,, sskkiinn,, dduurraa--mmaatteerr,, bboonnee,, lliiggaammeennttss Decellularization processes Viability of cells in fresh or Cryopreserved Allografts Cytotoxic preservatives Cross-linking Sterilizability and residuals Biodegradation Calcification Immune responses Biomechanical properties Infectious contamination- bacterial, viral, fungal, and prion
- 51. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Recently Approved Scaffold Biocompatibility testing of the ACI-Maix porcine membrane showed that the collagen membrane was not toxic or incompatible with biological tissue. In addition, the expansion process for chondrocytes did not induce changes to the cellular karyotype. MACI (autologous cultured chondrocytes on porcine collagen membrane) is an autologous cellularized scaffold product indicated for the repair of single or multiple symptomatic, full-thickness cartilage defects of the knee with or without bone involvement in adults.
- 52. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 53. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Malone, J, et al, 1984
- 54. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Nature Medicine Published online: 13 January 2008 Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart Harald C Ott1, Thomas S Matthiesen2, Saik-Kia Goh2, Lauren D Black3, Stefan M Kren2, Theoden I Netoff3 & Doris A Taylor2,4 About 3,000 individuals in the United States are awaiting a donor heart; worldwide, 22 million individuals are living with heart failure. A bioartificial heart is a theoretical alternative to transplantation or mechanical left ventricular support. Generating a bioartificial heart requires engineering of cardiac architecture, appropriate cellular constituents and pump function. We decellularized hearts by coronary perfusion with detergents, preserved the underlying extracellular matrix, and produced an acellular, perfusable vascular architecture, competent acellular valves and intact chamber geometry. To mimic cardiac cell composition, we reseeded these constructs with cardiac or endothelial cells. To establish function, we maintained eight constructs for up to 28 d by coronary perfusion in a bioreactor that simulated cardiac physiology. By day 4, we observed macroscopic contractions. By day 8, under physiological load and electrical stimulation, constructs could generate pump function (equivalent to about 2% of adult or 25% of 16-week fetal heart function) in a modified working heart preparation.
- 55. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Nature Medicine Published online: 13 January 2008 Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart Harald C Ott1, Thomas S Matthiesen2, Saik-Kia Goh2, Lauren D Black3, Stefan M Kren2, Theoden I Netoff3 & Doris A Taylor2,4 About 3,000 individuals in the United States are awaiting a donor heart; worldwide, 22 million individuals are living with heart failure. A bioartificial heart is a theoretical alternative to transplantation or mechanical left ventricular support. Generating a bioartificial heart requires engineering of cardiac architecture, appropriate cellular constituents and pump function. We decellularized hearts by coronary perfusion with detergents, preserved the underlying extracellular matrix, and produced an acellular, perfusable vascular architecture, competent acellular valves and intact chamber geometry. To mimic cardiac cell composition, we reseeded these constructs with cardiac or endothelial cells. To establish function, we maintained eight constructs for up to 28 d by coronary perfusion in a bioreactor that simulated cardiac physiology. By day 4, we observed macroscopic contractions. By day 8, under physiological load and electrical stimulation, constructs could generate pump function (equivalent to about 2% of adult or 25% of 16-week fetal heart function) in a modified working heart preparation.
- 56. Clinical transplantation of a tissue-engineered airway Paolo Macchiarini MD et al The Lancet, Early Online Publication, 19 November 2008 Bioengineered tubular tracheal matrices, using a tissue-engineering protocol, and to assess the application of this technology in a patient with end-stage airway disease. Removed cells and MHC antigens from a human donor trachea, which was then readily colonised by epithelial cells and mesenchymal stem-cell-derived chondrocytes that had been cultured from cells taken from the recipient (a 30-year old woman with end-stage bronchomalacia). This graft was then used to replace the recipient's left main bronchus. The graft immediately provided the recipient with a functional airway, improved her quality of life, and had a normal appearance and mechanical properties at 4 months. The patient had no anti-donor antibodies and was not on immunosuppressive drugs.
- 57. Wall Street Journal JUNE 25, 2010 Scientists Build a Rat Lung
- 58. 14 April 2013 Last updated at 19:29 ET Scientists make 'laboratory- grown' kidney By James Gallagher Health and science reporter, BBC News A kidney "grown" in the laboratory has been transplanted into animals where it started to produce urine, US scientists say Researchers at Massachusetts General Hospital have taken the first steps towards creating usable engineered kidneys. They took a rat kidney and used a detergent to wash away the old cells. The remaining web of proteins, or scaffold, looks just like a kidney, including an intricate network of blood vessels and drainage pipes. the membrane of collagen and sugars that remain are a perfect substrate for culturing cells and encouraging recellularization and functional tissue formation when stem and autologous (from the patient) cells are placed on this decellularized scaffold. http://www.bbc.co.uk/news/health- 22123386
- 59. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Bioderived Macromolecules Albumin, crosslinked Vascular graft coatings, ultrasound contrast agent, tissue adhesive Alginate Cell encapsulation, rapidly erodable biomaterial, e.g. urologic stent Cellulose acetates Hemodialysis membranes Cuprammonium cellulose Hemodialysis membranes Chitin and chitosans Experimental, coatings, controlled release Collagen Coatings, wound dressings, hybrid organs, tissue engineering scaffold, sealant, embolic Elastin Tissue engineering scaffold Fibrin Sealant, adhesive, bulking agent, tissue engineering scaffold Gelatin, crosslinked Coatings, tissue adhesive Hyaluronic acid Ocular and joint injectable, embolic, tissue engineering scaffold, anti-adhesive Phospholipids Liposomes, thromboresistant coating Silk Sutures, experimental coatings of silk-like protein
- 60. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Bioderived Macromolecules: eg albumin, Chitosans, collagen, gelatin, elastin, fibrin, hyaluronic acid, phospholipids, silk Purity Extractables Hydrolysis & Biodegradation Hypersensitivity reactions Lipid uptake Sterilization residuals Calcification Inflammatory and immune responses Permeability Water content Degree of cross-linking Effect of cross-linking on inflammation, immune response, and thrombogenicity Fatigue and wear particulates
- 61. Tissue engineered ecm’s and cells • Collagen/ecm sheets (grown in culture - eg smc and fibroblasts) • Cell layers onto ecm sheets/tubes • cultured endothelial cells
- 62. Cell based grafts cont.. • Ability to withstand over 2000mm Hg • 50% effective after implantation (1998) Uconn Tissue EngineeringBy Joanna Domka and Madeline Larkin
- 63. Bioink Composition Lifeink® 100 Type I Collagen, Methacrylated Lifeink® 200 Type I Collagen, Highly Concentrated Lifeink® 300 Gelatin, Methacrylated Lifeink® 400 Hyaluronic Acid, Methacrylated Native Material Bioinks For 3D Printing
- 64. Methacrylated Type I Collagen • Thermal gelation • UV/Visible light crosslinkable • Tunable construct strength Lifeink® 100
- 65. Lifeink® 200 Watch a Lifeink® 200 printed structure flex and recover like natural tissue. Concentrated Type I Collagen •Thermal gelation •Printed form integrity •Shear Thinning and Recovery •High resolution bioprinting •Tissue-like mechanics
- 66. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Passive Coatings Albumin Thromboresistance Alkyl chains Adsorbs albumin for thromboresistance Diamond-like coating Resistance to wear Fluorocarbons Reduced drag for catheters Hydrogels Reduced drag for catheters Ion beam modified surfaces Resistance to wear, reduced drag, controlled biologic interactions Plasma polymerized films Resistance to wear, reduced drag, controlled biologic interactions Silica-free silicones Thromboresistance Silicone oils Lubricity for needles and catheters Surface modifying agents Thromboresistance, biocompatibility Surface modifying groups at polymer end groups Thromboresistance, biocompatibility
- 67. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Passive Coatings: eg Albumin, diamond-like, fluorocarbons, hydrogels, PVD, CVD Adherence Wear Flaking Uniformity Sterilizability Shelf-life Durability Biostability Extractables Hypersensitivity Lipid uptake Calcification Sterilization residuals Fatigue
- 68. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Bioactive Coatings Anticoagulants, e.g., heparin Thromboresistance Antimicrobials Infection resistance Cell adhesion peptides Enhanced cell adhesion, endothelium Cell adhesion proteins Enhanced cell adhesion, endothelium Negative surface charge Thromboresistance Plasma polymerized coating Enhanced cell adhesion Thrombolytics Thromboresistance Tissue Adhesives Collagen/gelatin Coating, sealant, adhesive, embolic Cyanoacrylates Microsurgery for anastomosing vessels, adhesive, embolic Fibrin glue Coating, sealant, adhesive, embolic Polyethylene glycol Coating, sealant, adhesive, embolic Molluscan glue Enhancement of cell adhesion
- 69. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Bioactive Coatings (heparin, antimicrobials, cell adhesion peptides, surface charge) and Tissue Adhesives ( Collagen, cyanoacrylates, fibrin glue, PEG) Degree of antithrombogenicity, cell adhesion & tissue binding Biostability Wear & Durability Uniformity Sterilizability Microbiologic contamination Shelf-life Calcification Immune responses Residuals Tissue Adhesives - Purity - Filtration sterilization - Cure time - Tissue Adhesion
- 70. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Bioactive Molecules attached to surface
- 71. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 72. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 73. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com 30 minute exposure to canine blood Control (untreated) Duraflo® treated Bioactive Heparin Coatings Model for making a claim for a bioactive macromolecule
- 74. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Intramuscular Rabbit Implants 7 days Coating concentration > 0.5% DurafloTM Necrosis and increased host response compared to uncoated controls Coating concentrations< 0.3 % Biocompatible Helmus, Michael N., Scott, Michael J., Enhanced Biocompatibility Coatings for Medical Implants, WO99/38547, Aug. 5, 1999 Ex Vivo Canine Shunt 100 ml/min flow Bioactive Heparin Coatings Edwards Duraflo coated Annuloplasty Rings
- 75. •7,625,552 Bioactive polymers for imparting bioactive character to hydrophobic medical article surfaces •20070269480 Medical devices having bioactive surfaces •7,709,439 Biomaterials for enhanced healing •20040093080 Bioactive coatings to prevent tissue overgrowth on artificial heart valves Bioctive coatings for enhanced healing
- 76. A biomaterial comprising: a bioactive polymer comprised of at least one peptide and/or protein subunit and at least one polysaccharide and/or proteoglycan subunit; and a biocompatible polymer. …polysaccharide and/or proteoglycan subunit of the bioactive polymer is selected from the group consisting of aggrecan, agrin, bamacan, heparan sulfate, chondroitin sulfate, keratan sulfate, perlecan, hyaluronan, decorin, dermatan sulfate, biglycan, fibromodulin, alginate, polylactate, polyglycolic acid, starch, dextran, agarose and heparin. … cell adhesion peptide is a RGD peptide, a dRGD peptide, a YIGSR peptide or a IVKAV peptide …peptide and/or protein subunit of the bioactive polymer is a growth factor protein. …growth factor protein is insulin, insulin like growth factors, interleukin-4, platelet derived growth factor, TGF-.beta., EGF, NGF, IL-2, II-3, VEGF, GM-CSF, M-CSF, G-CSF, EPO or FGF. Biomaterials for enhanced healing
- 77. Example: RGD Peptides Endothelial Cell adhesion to Synthetic Surfactant Bound RGD Peptides Surfactant Bound RGD Hydrophobic (Leucine) Rich polypeptide tail. Peptite 2000
- 78. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Medical Textile Modified with Cell Adhesion Peptide RGD Coated Polyester and PTFE Fabric Effect on Cell Adhesion In vivo healing of fabric K. Tweden, Chapter 8 in "Biomaterials in the design and reliability of medical devices", M. N. Helmus, ed., Landes Bioscience, Georgetown, TX, 2001 Tweden KS, Haraskai H, Jones M, Blevitt JM, Craig WS, Pierschbacher M, Helmus M, Accelerated healing of cardiovascular textiles promoted by an RGD peptide, J. Heart Valve Dis 1995; 4 (Suppl. I):S90-97
- 79. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com K. Tweden, Chapter 8 in "Biomaterials in the design and reliability of medical devices", M. N. Helmus, ed., Landes Bioscience, Georgetown, TX, in press. PETControl PepTite 2000 RGDCoating RGD Peptide Coating: In Vivo Response PET Arterial Patch Canine Implants at 3 weeks
- 80. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com K. Tweden, Chapter 8 in "Biomaterials in the design and reliability of medical devices", M. N. Helmus, ed., Landes Bioscience, Georgetown, TX, in press.
- 81. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 82. •Ceramic but with catalytic properties • Catalytic properties of the oxide converts H202 to water and oxygen, reducinginflammatory responses •Successful history on pacemaker electrodes •Minimal proliferation of smooth muscle cells 30 daysBarry O’Brien, Boston Scientific Active Stent Coatings Iridium Oxide
- 83. • Pre-clincal studies show that iridium oxide facilitates endothelialization and reduced inflammatory response • Minimal proliferation of smooth muscle cells 30 days Barry O’Brien, Boston Scientific Active Stent Coatings Iridium Oxide
- 84. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Metals and Metallic Alloys Cobalt chrome alloys Nickel chrome alloys Nitinol alloys (shape memory alloys) Stainless steels Tantalum Titanium and titanium alloys Guide wires, mechanical heart valve orifices and struts, biologic heart valve stents, endovascular and urologic stents, vena cava umbrellas, orthopedic and dental implants, artificial heart housings, pacemaker leads Platinum/iridium Tungsten Radioopaque markers, radioopaque coils for guidewires Ceramics, Inorganics, and Glasses Bioglasses Bone attachment, reconstructive surgery Bioactive glass/ceramics Bone attachment, reconstructive surgery Hi-density alumina Orthopedic and dental implants Hydroxyapatite Bone attachment, reconstructive surgery Nanocrystalline brushite Biodegradable for drug delivery Single crystal alumina Orthopedic and dental implants Tricalcium phosphates Bone repair Zirconia Bone attachment, reconstructive surgery
- 85. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Metals and Metallic Alloys Passive layer durability Corrosion - pitting, fretting, stress Corrosion by-products Fracture toughness Fatigue life Stiffness compared to application Porous coatings Hypersensitivity Noble metal protein interactions; Antimicrobial activity, eg. Ag, Cu Wear Ceramics, Inorganics, and Glasses Bioactivity and Degree of bone formation Bioresorption rate Biostability Biodegradation by-products Fatigue and wear particulates
- 87. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 88. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Bioactive Nature of Titanium Oxide Surfaces
- 89. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 90. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Carbons Pyrolytic (low temperature isotropic) carbon Heart valves, coatings for cardiovascular implants Ultra low temperature isotropic carbon Coatings on heat sensitive polymers Composites Carbon fiber composites based on a matrix material of: Epoxy Poly(etherketone s) Poly(imide) Poly(sulfone) Potential materials for orifices, disks, and stents, orthopedic implants Radioopacifiers (BaSO4, BaCl2, TiO2) blended into polymers of: Poly(olefins) Poly(urethanes) Silicones Radioopaque on x-ray for identifying location of the device
- 91. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Polymethylmethacryla te bone cements Radioopaque bone cement HA fiber composites based on a matrix material of polylactic acid copolymers High-strength biodegradable bone plates and rods Polymethylmethacryla te bone cements with HA particles Bone cement with enhanced bone formation at the interface Bis-GMA bone cements with HA particles Bone cement with enhanced bone formation at the interface Collagen/HA particle Bone repair Plaster of Paris/ hydroxyapatite particle bone filler Bone repair Calcium sodium metaphosphate short fiber reinforced biodegradable polymer Bone and soft tissue repair
- 92. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Carbons: Pyrolytic, Ultra low temp. isotropic Wear resistance Biostability Low heat of protein adsorption Thromboresistance Fatigue Composites: Carbon fiber, nanoparticles, radioopacifiers Surface exposure of compounded particles Extractables Hypersensitivity Residuals Lipid uptake Hydrolytic stability Biostability Biodegradation by-products Calcification Sterilization residuals Fatigue and particulates
- 93. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Enhanced biocompatibility for Regenerative Medicine is a Disruptive Medical Technology
- 94. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Technologies that introduce a different performance package than mainstream technologies and are inferior to mainstream technologies… Technology disruption occurs when, despite its inferior performance, the new technology displaces mainstream technology from the mainstream market RON ADNER , WHEN ARE TECHNOLOGIES DISRUPTIVE? A DEMAND-BASED VIEW OF THE EMERGENCE OF COMPETITION Strat. Mgmt. J. (in press) Disruptive Technology
- 95. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Enhanced Functionality Enhanced Biocompatibility Anti-infectives Thromboreistant Reduced inflammation Reduced hyperplastic response Recellularizaion Neogenesis of Tissue Enhanced Biomechanics Improved biocompatibility by matching tissue properties
- 96. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Surface Properties and Biologic Interactions • Surface Energy • Polar, Dispersion, Hydrogen bonding interactions • Critical surface energy • Wettability • Surface heterogeneity –Micro –Nano • Surface texture •Micron vs nano • Surface Mobility • Hydrogel surfaces • Grafted surfaces • Bioactive Surfaces
- 97. Examples of Emergent Biomaterials to control wound healing and Inflammation
- 98. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com TEM micrograph of random, irregularly shaped polystyrene domains (white phase) in a polybutadiene matrix. Stained with osmium tetroxide, Scale bar = 100 nm. M. Helmus et al., Adv. Chem. Series, No. 199, 1982, pp. 81-93. Reprinted with permission American Chemical Society.
- 99. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com TEM micrograph of ordered, spherically shaped polystyrene domains (white phase) in a polybutadiene matrix. Stained with osmium tetroxide, Scale bar = 100 nm. M. Helmus et al., Adv. Chem. Series, No. 199, 1982, pp. 81-93. Reprinted with permission American Chemical Society.
- 100. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Effect of Mircophase Separation in SBS on Fibrinogen Conversion. (Reprinted with permission from (5), copyright 1982 American Chemical Society). Material Normalized Platelet Adhesion % Conversion 3 sec. Plasma Exposure 3 min. Plasma Exposure SBS random 102 + 30 45 + 26 56 SBS ordered 97 + 24 64 + 18 34 Hydrophobic Glass Control 104 + 25 100 + 31 4 Hydrophilic Glass Control 79 + 5 1 + 1 99
- 101. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Commercializing New Technology: Development Cycle Start Preclinical /Clinical Animal testing IDE/IND Human Clinical Concept Prototype Quality Systems Packaging CMC Chemistry Manufact. Controls Sterility Inventory Marketing Epidemiology, Adverse Reporting, Post-Market Surveillance Toxicology, Hazard Analysis, Study Design, Statistics Toxicokinetics Pharmacology, Pharmacokinetics, ADME, Biocompatibility FMEA Design Freeze National Materials Components Technology Pharma Biologics Modified from Helmus, Nature Nanotechnology 1, 157 - 158 (2006) 510K, PMA, NDA
- 102. COMBINATION MEDICAL DEVICE VALUE CHAIN • Powders • Dispersions • Coatings • Composites • Biomaterials • Proteomics • Genomics Technology Medicine Develop IP Strategy: Composition of Matter Applications File IP
- 103. Tissue Eng Part B Rev. 2010 Feb;16(1):41-54. doi: 10.1089/ten.TEB.2009.0449. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States.
- 104. The development of efficacious therapeutic and diagnostic procedures based on nanotechnology will require the early collaboration of clinicians and an understanding of the clinical environment Nanomedicine
- 105. The Promise and the Challenge of Nano-enabled technologies for Medical Applications •Enhanced functionality and biocompatibility •Potential new paradigms required for biocompatibility evaluations of nano- structures and particles
- 106. NANOMATERIALS AND PROPERTIES BIOMIMETICS BIOACTIVE Metal ceramic fiber Ceramic metal filled Polymer nanoparticulates Polymer nanofibers Polymer-layered silicate nanocomposite(PLSN) Polyelectrolyte layered nanocomposites NANOPARTICLES NANOCOMPOSITES NANOSTRUCTURED BULK, COATINGS & SURFACES NANOPOROUS DRUGDELIVERY DIAGNOSTICS Bottom up: CVD, PVD, Deposition processes by Laser, EBeam Self Assembly, Sintering Top down: Nanolithography, Nanomachining, Ablation, Dissolution BIOSENSORS MICROFLUIDICS Microphase separated polymers Nanograined ceramics & metals Self-assembled monolayers Surface nano-clusters SWNT Fibers Polymeric Fibers and yarns Nanowires Nanotubes – Carbon, BN, Metallic Metal Nanoribbons Quantum Dots Dendrimers Gold and metallic nanoshells Buckminister Fullerenes Paramagnetic nanoparticles PHOTONICS SEMICONDUCTO RS STRUCTURAL
- 107. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 108. Biocompatibility Evaluation and Issues
- 109. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Tissue Engineered Devices ASTM Committee F04 Tissue Engineered Medical Products F2211-13 Standard Classification for Tissue Engineered Medical Products (TEMPs) F2312-11 Standard Terminology Relating to Tissue Engineered Medical Products F3163-16 Standard Guide for Classification of Cellular and/or Tissue-Based Products (CTPs) for Skin Wounds
- 110. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com F2150-13 Standard Guide for Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products F2027-16 Standard Guide for Characterization and Testing of Raw or Starting Materials for Tissue-Engineered Medical Products F2450-10 Standard Guide for Assessing Microstructure of Polymeric Scaffolds for Use in Tissue Engineered Medical Products F2103-11 Standard Guide for Characterization and Testing of Chitosan Salts as Starting Materials Intended for Use in Biomedical and Tissue-Engineered Medical Product Applications F2903-11 Standard Guide for Tissue Engineered Medical Products (TEMPs) for Reinforcement of Tendon and Ligament Surgical Repair F2883-11 Standard Guide for Characterization of Ceramic and Mineral Based Scaffolds used for Tissue-Engineered Medical Products (TEMPs) and as Device for Surgical Implant Applications
- 111. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com
- 112. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com INTERNATIONAL STANDARDS FOR MEDICAL DEVICES Part 1: Evaluation and testing (ANSI/AAMI/ISO 10993-1:2009) Part 2: Animal welfare requirements, (ANSI/AAMI/ISO 10993-2:2006) Part 3: Tests for genotoxicity, carcinogenicity, and reproductive toxicity, 2ed (ANSI/AAMI/ISO 10993-3:2003) Part 4: Selection of tests for interactions with blood (ANSI/AAMI/ISO 10993-4:2002(R)2009 & A1:2006(R)2009) Part 5: Tests for in vitro cytotoxicity, 3ed (ANSI/AAMI/ISO 10993-5:2009) Part 6: Tests for local effects after implantation, 2ed (ANSI/AAMI/ISO 10993-6:2007) Part 7: Ethylene oxide sterilization residuals, 3ed (ANSI/AAMI/ISO 10993-7:2008 Part 9: Framework for identification and quantification of potential degradation products, 2ed (ANSI/AAMI/ISO 10993-9:2009) Part 10: Tests for irritation and delayed type hypersensitivity, 2ed (ANSI/AAMI BE78:2002 (R)2008; adoption of ISO 10993-10:2002 with national deviation) Part 11: Tests for systemic toxicity (ANSI/AAMI 10993-11:2006) Part 12: Sample preparation and reference materials, 3ed (ANSI/AAMI/ISO 10993-12:2007) Part 13: Identification and quantification of degradation products from polymeric medical devices, 1ed (ANSI/AAMI/ISO 10993-13:1999/(R)2004) Part 14: Identification and quantification of degradation products from ceramics, 1ed (ANSI/AAMI/ISO 10993-14:2001/(R)2011) Part 15: Identification and quantification of degradation products from metals and alloys, 1ed (ANSI/AAMI/ISO 10993-15:2000/(R)2011) Part 16: Toxicokinetic study design for degradation products and leachables, 2ed (ANSI/AAMI/ISO 10993-16:2010)
- 113. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Part 17: Establishment of allowable limits for leachable substances, 1ed (ANSI/AAMI/ISO 10993-17:2002(R)2008 Part 18:Chemical characterization of materials (ANSI/AAMI BE83:2006/(R)2011) Part 19: Physicochemical, morphological, and topographical characterization of materials (ANSI/AAMI/ISO 10993-19:2006) Part 20: Principles and methods for immunotoxicology testing of medical devices (ANSI/AAMI/ISO 10993-20:2006) ---------------------------------------------------------------------------------------------------------------- -Clinical investigation of medical devices for human subjects (ANSI/AAMI/ISO 14155:2011 -Guidance for ANSI/AAMI/ISO 10993-7:1995, Biological evaluation of medical devices-Part 7: Ethylene oxide sterilization residuals, 1ed and Amendment (AAMI TIR19:1998; TIR19/A1:1999) -22442-1:2007/(R)2011, Medical devices utilizing animal tissues and their derivatives - Part 1: Application of risk management -22442-2:2007/(R)2011, Medical devices utilizing animal tissues and their derivatives - Part 2: Controls on sourcing, collection and handling -22442-3:2007/(R)2011, Medical devices utilizing animal tissues and their derivatives - Part 3: Validation of the elimination and/or inactivation of viruses and transmissible spongiform encephalopathy (TSE) agents -22442-4:2010, Medical devices utilizing animal tissues and their derivatives -- Part 4: Principles for elimination and/or inactivation of transmissible spongiform encephalopathy (TSE) agents and validation assays for those processes
- 114. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Future Directions: Tissue Engineering Approaches Self Reparative Masked Immune Response No Calcification No Anti-Coagulation Unlimited Supply Bioreactor Recellularization In Situ Recellularization Platform Bioresorbable Exracellular- Scaffolfds Matrix (ECM) Cell Therapy
- 115. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Tissue Engineering: The First Decade and Beyond Lawrence J. Bonassar* and Charles A. Vacanti Journal of Cellular Biochemistry Supplements 30/31:297–303 (1998)
- 116. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Implantation of tissue engineered construct. Tissue Engineered Devices Biopsy/tissue sample for autologous cell &/or isolation of stem cells. Culturing of cells to expand if needed. Scaffolds: Decellularized tissue, Polymer, Biodegradables, Bioderived, eg collagen Seeded scaffolds for direct implantation or growth of tissue in bioreactor Healed device
- 117. Michael N. Helmus, Ph.D., Consultant mnhelmus@msn.com Small Molecules Proteins/Growth Factors Gene Transfection Remodeled Organ In Situ Healing Injectables to recruit bmc’s/tissue stem cells to Regenerate in situ.
- 118. Biomaterials in Sustainability of Tissue Engineering Michael N. Helmus, Ph.D., Consultant Medical Devices, Biomaterials, Drug Delivery, and Nanotechnology mnhelmus@msn.com
