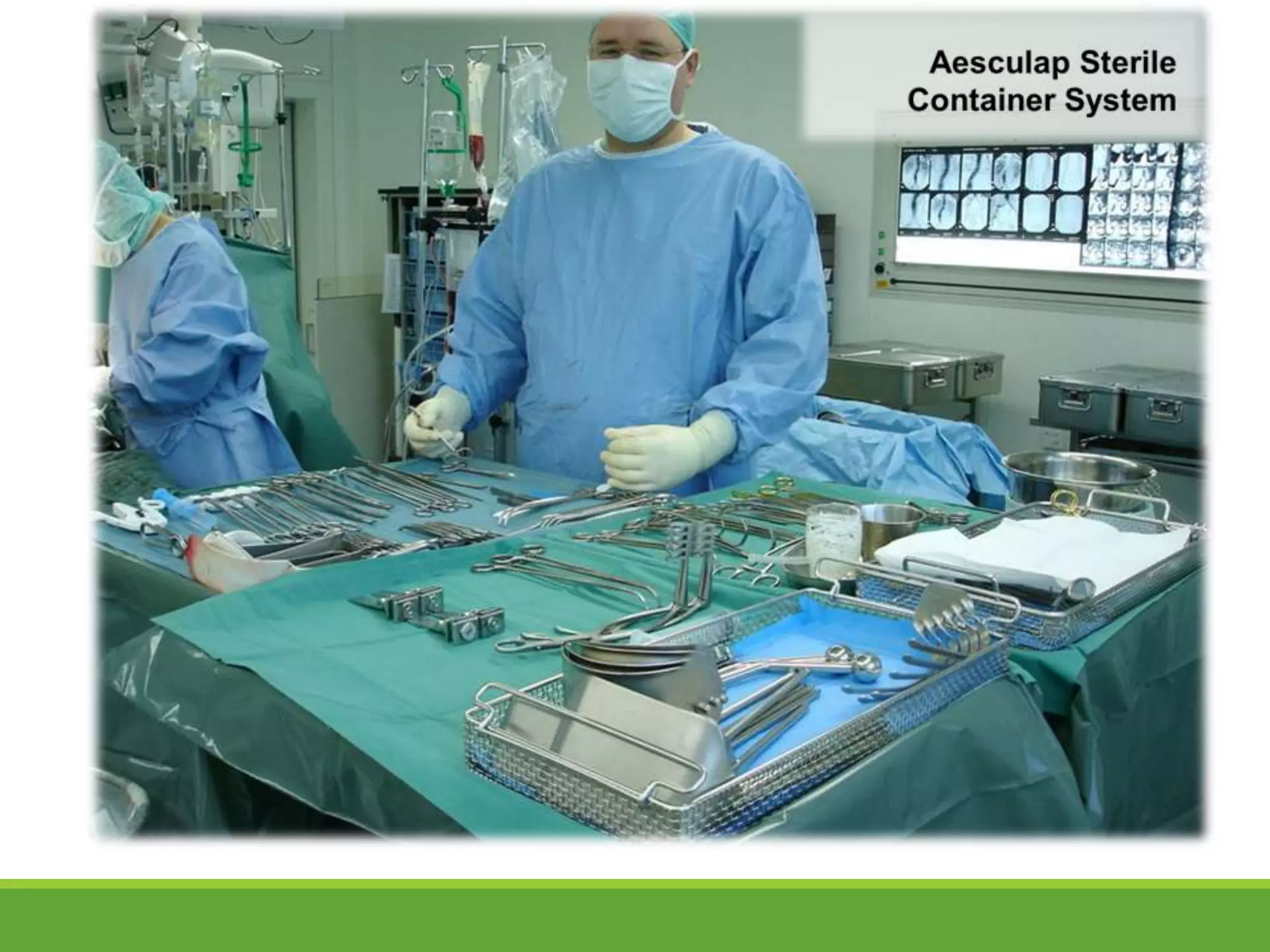
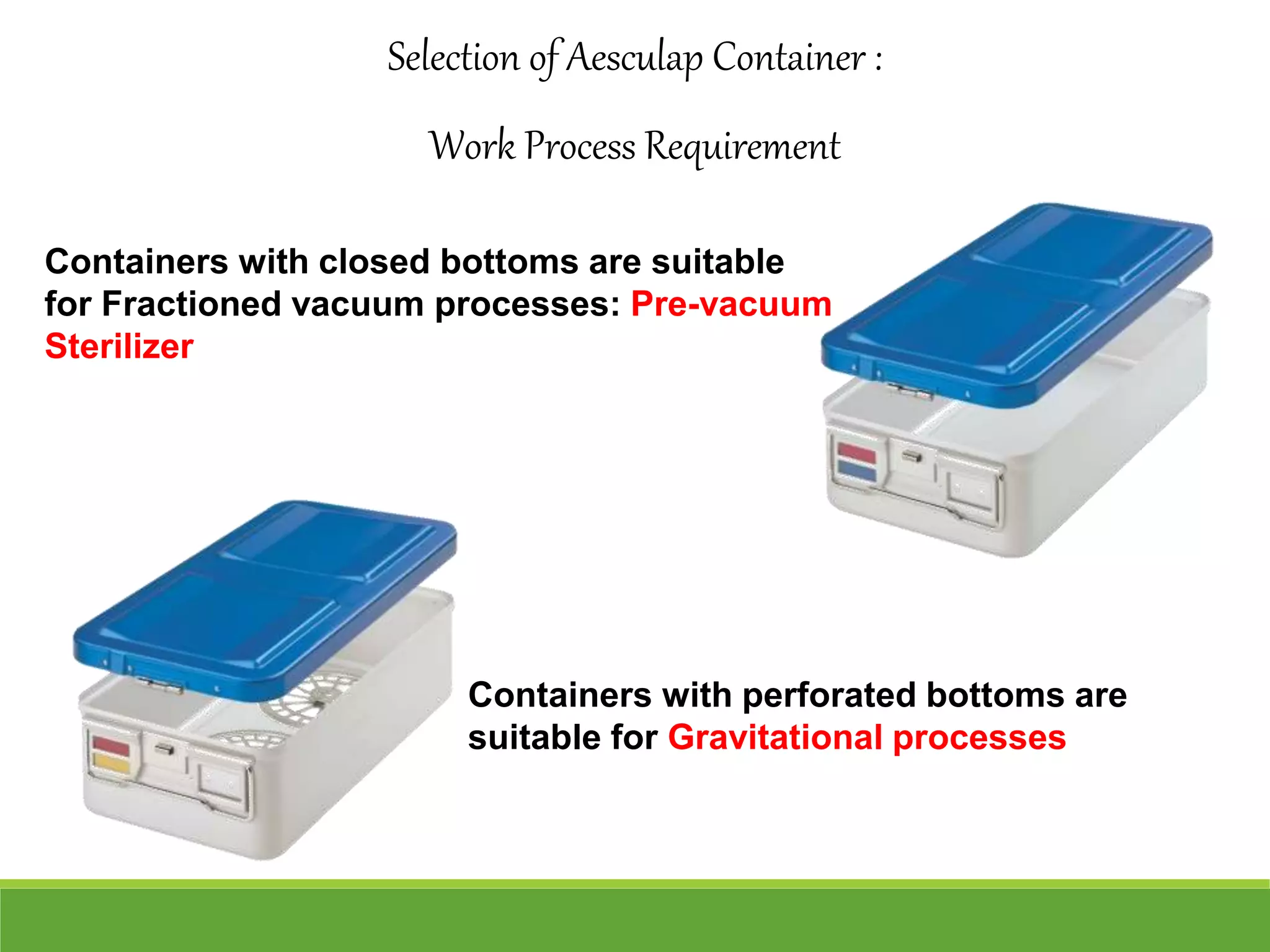
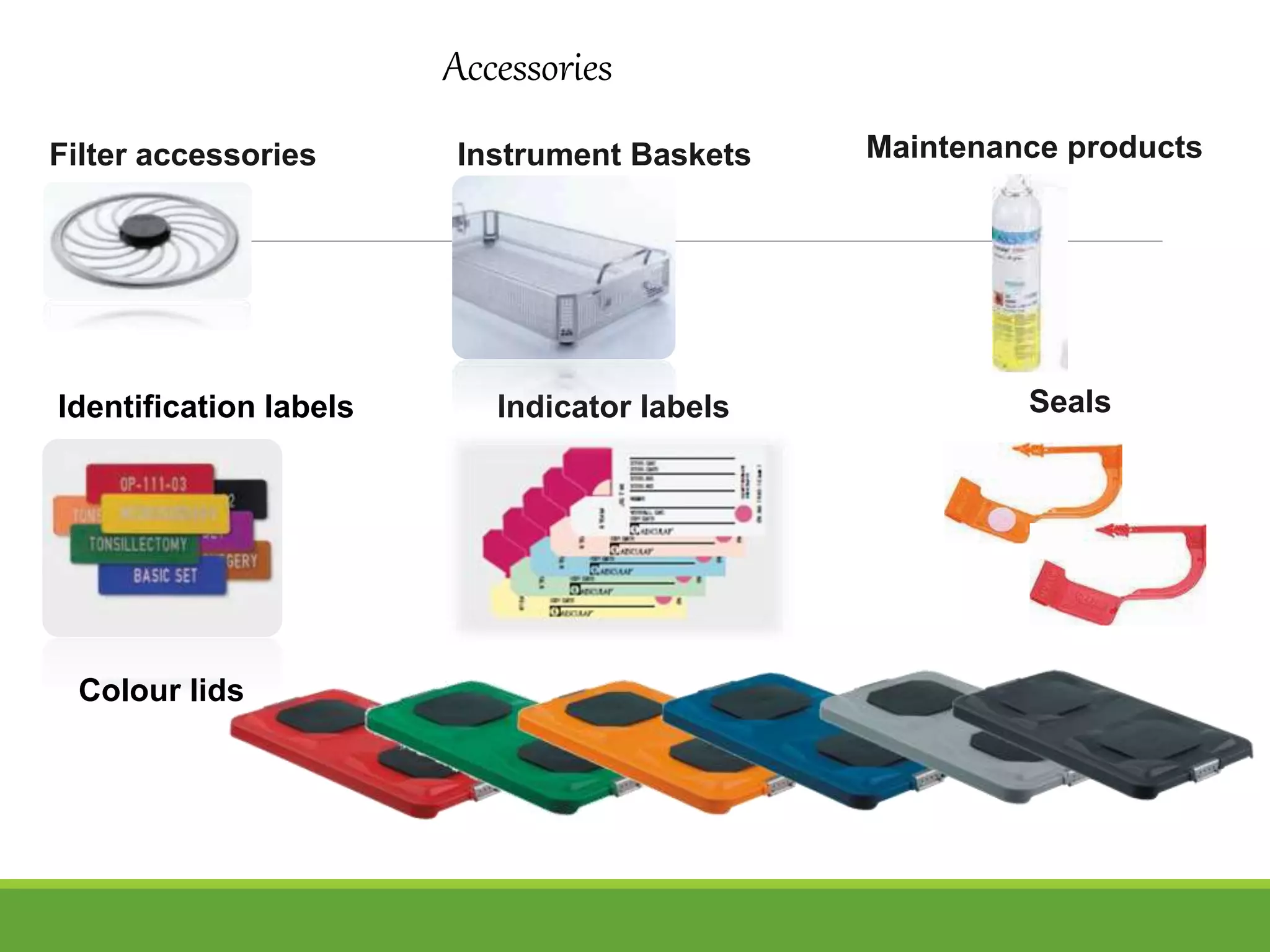
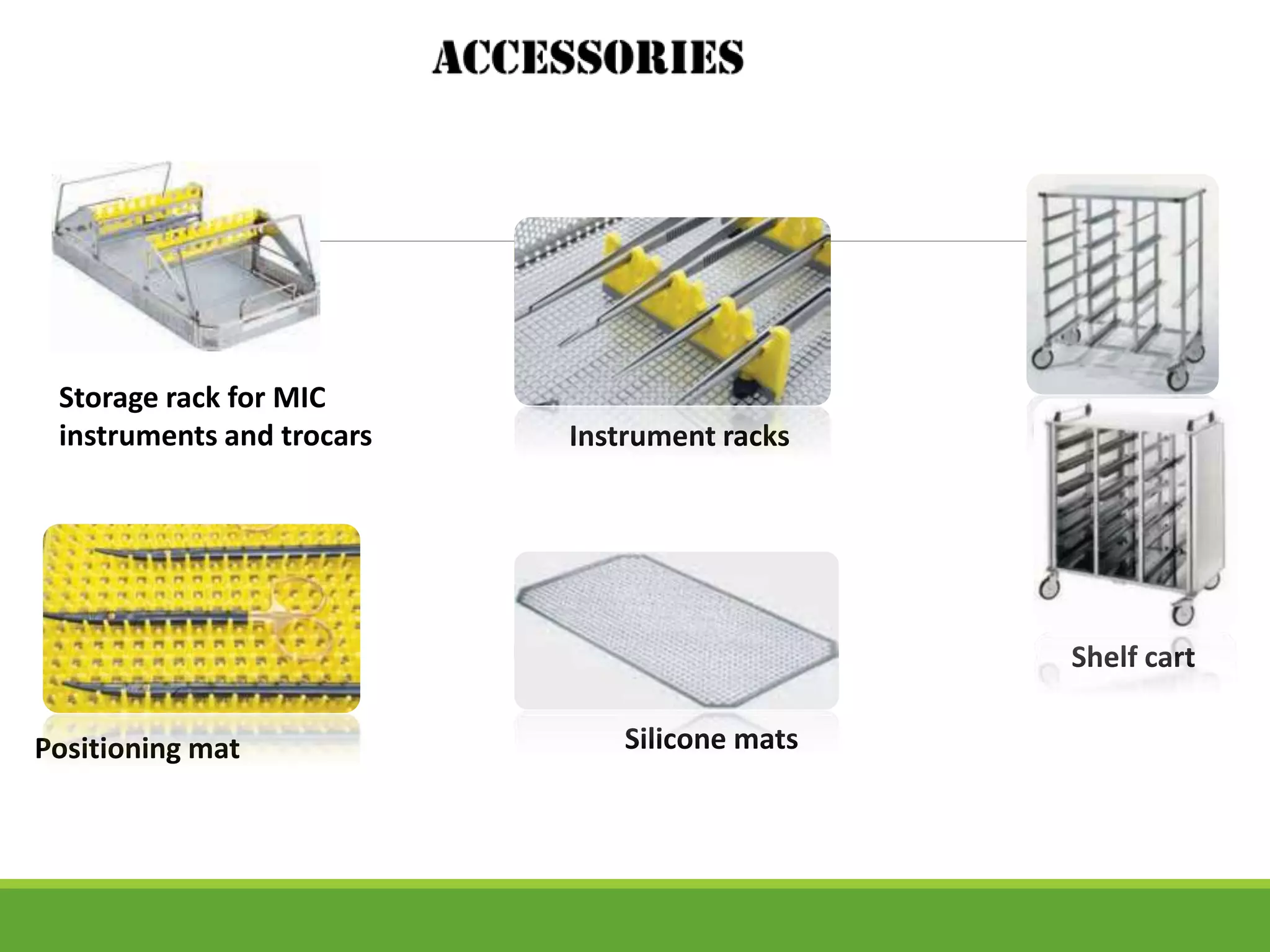
Surgical site infections are a common complication in postoperative care, and ensuring the sterility of surgical instruments is crucial for prevention. Aesculap's sterile container system meets various requirements, including maintaining sterility during transport and handling, and facilitating aseptic presentation. The system includes features such as protective packaging, compatibility with medical products, and functional tests to ensure the integrity and effectiveness of sterile containers.