The lithium ion fire problem versus the lithium metal and graphene battery
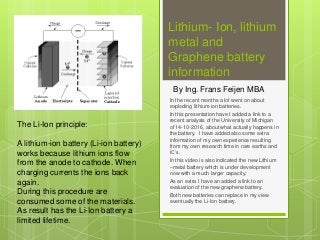
- 1. Lithium- Ion, lithium metal and Graphene battery information In the recent months a lot went on about exploding lithium-ion batteries. In this presentation have I added a link to a recent analysis of the University of Michigan of 14-10-2016, about what actually happens in the battery. I have added also some extra information of my own experience resulting from my own research time in rare earths and IC’s. In this video is also indicated the new Lithium –metal battery which is under development now with a much larger capacity. As an extra I have an added a link to an evaluation of the new graphene battery. Both new batteries can replace in my view eventually the Li-Ion battery. By Ing. Frans Feijen MBA The Li-Ion principle: A lithium-ion battery (Li-ion battery) works because lithium ions flow from the anode to cathode. When charging currents the ions back again. During this procedure are consumed some of the materials. As result has the Li-Ion battery a limited lifetime.
- 2. Analysis of the the actual problem of the Lithium-ion battery http://www.nanowerk.com/nanotechnology- news/newsid=44844.php?utm_source=feedblitz&utm_medium=FeedBlitzRss&utm_campaign=nanowe rkemergingtechnologiesnews Please follow below link or copy it into your browsers address bar. It is aboyut the recent analysis of the Li-Ion problem by the University of Michigan (14-oct-2016). It also contains information about the Lithium-metal battery. The analysis
- 3. Process evalaution with help of the Michigan dendrite formation video (By Ing. Frans Feijen MBA) The video confirms myknowledge in rare earth’s and electro migration of material. The explosion process starts with the formation of Lithium dendrites based on an electro migration process which is initiated by the voltage difference over the battery electrolyte. This formation s clearly shown in the video of the University of Michigan (very good analysis by the way). It is actually the same process which happens if you use silver in an IC: the silver migrates slowly over none silver containing area’s and eventually formats a shortage wire. This shortage wire releases the voltage differences between the 2 connected areas. This is initially a small difference but during the shortage becomes this shortly a very high voltage (typically 15.000 - 60.000 volts) which is released as and arc. One can compare this with an electrical arc flash in a thunderstorm. During the arc-flash is the electrolyte fluid of the battery transformed into a gas. The expansion of the fluid into gas is shown by the fact that the battery is blown-up like a balloon. This goes on for some time until the battery edge is overstressed and opens. After this comes the lithium in motion: lithium is extremely corrosive and powder and materials need to be handled in oxygen free environments. The battery environment is however compromised now and oxygen pours in. We have now a corrosive material (lithium + electrolyte) with an ignition point (the arc) which can get into and explosive fire as result of the pouring in oxygen. The fire triangel is complete: A battery explosion occurs as is shown in the video. I can indicate the following from my own research knowledge in rare earth’s and IC manufacturing The Process
- 4. How can a fire or expolosion be prevented? This can only prevented if the dendrites can’t grow, the electrolyte can’t evaporate or the oxygen can’t enter the battery. Possible options: A containment layer around the Lithium An electrolyte which is less conducting, solid and can’t sublimate. A battery material which is less corrosive An electrical loading procedure which is less fast or at least does not initiate the dendrite formation. A combination of all 4 can create a battery in which dendrites do not grow and no fire hazard can occur. To my opinion (Frans Feijen) can I indicate the following now: if there isn’t looked at all 4 simultaneously, then shall this problem not be solved. The solutuion
- 5. What can be said further about at the lattest battery developments? There is looked at this moment at 2 new types of batteries. Knowing: 1) Lithium-metal batteries (Also mentioned in the video of the University of Michigan) 2) Graphene batteries (Please look at the video at the next page.) Both materials still can still ignite/oxidize if arc’s can be formed and oxygen is added. Graphene is however certainly less oxidizing than Lithium. The wish for fast loading of the batteries does fuel the possibility of dendrite formation and shortages. In my view we only can avoid Lithium dendrite formation if the loading can be done via a sort of alternation current and a creation of a sophisticated loading protocol. The future
- 6. Some Information about The graphene battery https://youtu.be/8A5UPTWno4c Please follow below link or copy it into your browsers address bar and look what is avaliable about the new Graphene Batteries: There can be found more video’s and information on youtube and the internet about this type of batteries. Graphene battery
