Chapter13 140331231310-phpapp01
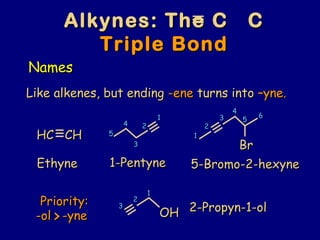
- 1. Like alkenes, but endingLike alkenes, but ending -ene-ene turns intoturns into –yne.–yne. HC CHHC CH EthyneEthyne 55 44 33 22 11 1-Pentyne1-Pentyne BrBr 11 22 33 44 55 66 5-Bromo-2-hexyne5-Bromo-2-hexyne -ol -yne-ol -yne>> OHOH 11 22 33 2-Propyn-1-ol2-Propyn-1-ol Alkynes: The C CAlkynes: The C C Triple BondTriple Bond NamesNames Priority:Priority:
- 2. When the alkyne contains also double bonds, itWhen the alkyne contains also double bonds, it is called anis called an enyne.enyne. However, despite being anHowever, despite being an “yne”, numbering begins closest to either group:“yne”, numbering begins closest to either group: 11 22 33 44 55 66 3-Hexen-1-yne3-Hexen-1-yne 1-Penten-4-yne1-Penten-4-yne 11 22 33 44 55 When double and triple bond are equidistant fromWhen double and triple bond are equidistant from each terminus:each terminus: Ene firstEne first (alphabetical)(alphabetical) 11 22 33 44 55 66 77 1-Hepten-4-yne1-Hepten-4-yne
- 3. Substituents:Substituents: EthynylEthynyl 2-Propynyl2-Propynyl (or propargyl)(or propargyl) Rings:Rings: Naming follows hydrocarbon rule:Naming follows hydrocarbon rule: Smaller R is a substituent to larger R (ignoreSmaller R is a substituent to larger R (ignore function)function) 3-Cyclobutyl-1-hexyne3-Cyclobutyl-1-hexyne EthynylcyclohexaneEthynylcyclohexane 11 22
- 4. Two perpendicularTwo perpendicular ππ bonds;bonds; spsp hybridshybrids RR CC CC RR EthyneEthyne
- 6. The Triple Bond isThe Triple Bond is EnergeticEnergetic Kcal molKcal mol-1-1 Heat of hydrogenationHeat of hydrogenation:: MoreMore than Two Alkenethan Two Alkene ππ BondsBonds (which would be ~ -60 kcal mol(which would be ~ -60 kcal mol-1-1 )) DDHH ° HC CH H° HC CH H22C CHC CH22 HH33C CHC CH33 229229 173173 9090
- 8. Hydrogens get more acidicHydrogens get more acidic (blue)(blue) Acidity:Acidity: RC CRC C HH ++ BB R CR C CC ++ HHBB -- -- :: :: ppKKaa ~ 25!~ 25! cf. CHcf. CH22 CHCH22 44, CH44, CH33 CHCH33 5050 Why?Why? 50% s-character50% s-character KK
- 9. ++ LiLi oror LiLi LiLi ++-- ++ ++ CHCH33MgBrMgBr MgBrMgBr ++ CHCH44 ppKKaa 2525 ppKKaa 5050 H H + Na NHH H + Na NH22 ++ :: :: HH NaNa C CC C:::: ---- NHNH33++ NaNHNaNH22 NaNa ++ ++ ppKKaa 3333 1 equiv.1 equiv. ::-- Synthetic Use ofSynthetic Use of AcidityAcidity --
- 10. 11 H NMR:H NMR: RC CRC C HH δδ = 1.7-3.1 != 1.7-3.1 ! Recall:Recall: RCH CHRCH CH22 δδ = 4.6-6 ppm= 4.6-6 ppm
- 11. AlkAlkeneene hydrogens:hydrogens: deshieldeddeshielded Why?Why? Cylindrical electron currentCylindrical electron current shields alkynyl hydrogenshields alkynyl hydrogen AlkAlkyneyne hydrogens:hydrogens: shieldedshielded Recall alkene NMRRecall alkene NMR
- 12. Long range:Long range: RCRCHH22 C CC C HH JJ = 2-4 Hz= 2-4 Hz RCRCHH22 C C CC C CHH22 R’R’ JJ = 2-3 Hz= 2-3 Hz Coupling:Coupling:
- 14. 1313 C NMR:C NMR: δδ = 65-85 ppm: Also= 65-85 ppm: Also shielded.shielded. Compare alkenes:Compare alkenes: 120-150ppm.120-150ppm. HC CCHHC CCH22CHCH22CHCH22CHCH33 14-3114-316969 8484 IR spectra:IR spectra: diagnostic peaks for triple bonddiagnostic peaks for triple bond and its attached H.and its attached H. υυ (R(RC CC CR’) =R’) = 2120 cm2120 cm-1-1 ;; υυ (RC(RC C HC H) =) = 3300 cm3300 cm-1-1~~ strongstrong ~~
- 16. Stability of Alkynes:Stability of Alkynes: Heats of HydrogenationHeats of Hydrogenation RevisitedRevisited CHCHHCHC ++ HH22 Special cat.Special cat. CHCH22 CHCH22 ΔΔHH ° = -44.9° = -44.9 kcal molkcal mol-1-1 CHCH22 CHCH22 ++ HH22 Cat.Cat. CHCH33 CHCH33 ΔΔHH ° = -32.7° = -32.7 kcal molkcal mol-1-1 FirstFirst ΠΠ bond has more “heat content”, isbond has more “heat content”, is also more reactive. Allows for:also more reactive. Allows for: RR11 CC CC RR22 ++ A BA B AA CC CC RR22 RR11 BB AA CC CC BB RR11 RR22 ++ Stereoselective alkene synthesisStereoselective alkene synthesis
- 17. InternalInternal alkynes arealkynes are more stablemore stable thanthan terminal onesterminal ones ++ HH22 cat.cat. ++ HH22 cat.cat. ΔΔHH ° = -69.9 kcal mol° = -69.9 kcal mol-1-1 ΔΔHH ° = -65.1 kcal mol° = -65.1 kcal mol-1-1 Parallels the behavior of alkenes.Parallels the behavior of alkenes. Same reason:Same reason: hyperconjugationhyperconjugation..
- 18. PreparationPreparation 1.1. Elimination E2 of DihaloalkanesElimination E2 of Dihaloalkanes CC CC HH HH XX XX CC CC CC CC BrBr BrBr NaNa B:B:-- HH XX B:B:-- NaNHNaNH22 excessexcess NHNH33 liq.liq. HH++ , H, H22OO work upwork up 75%75%
- 19. Application in synthesis:Application in synthesis: RCH CHR R C C RRCH CHR R C C R BrBr BrBrBrBr BrBr BrBr22 NaNHNaNH22 NHNH33 liqliq 1,5-Hexadiyne1,5-Hexadiyne
- 20. 2. Alkylation of Alkynyl Metals2. Alkylation of Alkynyl Metals SSNN2 rules2 rules LiLi THFTHF LiLi II ∆∆ 90%90% BestBest: RI, THF, ∆. RBr or RCl need: RI, THF, ∆. RBr or RCl need “coordinating”“coordinating” additives: e.g. ;additives: e.g. ; or HMPA. Remember: Grignardsor HMPA. Remember: Grignards don’t workdon’t work for RX, but O.K. for orfor RX, but O.K. for or carbonyls.carbonyls. HH22NN NHNH22 OO
- 21. + CH+ CH33MgBrMgBr MgBrMgBr CHCH22 OO OHOH ++ LiNHLiNH22 (l equiv)(l equiv) LiLi OO OHOH + 2 CH+ 2 CH33MgBrMgBr MgBrMgBrBrMgBrMg HOHOOHOH LiLi++ LiLi OO CHCH33 OHOH CHCH33CHCH OO
- 22. ReactionReaction ss 1. Reductions1. Reductions a. Complete hydrogenationa. Complete hydrogenation HH22, Pt, Pt 100%100% b. Partial hydrogenation:b. Partial hydrogenation: “Poisoned”“Poisoned” Lindlar’sLindlar’s catalyst:catalyst: Cis!Cis! Pd-CaCOPd-CaCO33, Pb(OCCH, Pb(OCCH33))22, quinoline, quinoline OO NN HH22,, LindlarLindlar 100%100% HHHH CisCis-3-heptene-3-heptene c. Na reduction:c. Na reduction: Trans!Trans! Via stepwise 2e transferVia stepwise 2e transfer + Na°+ Na° NNHH33 liq.liq. 86%86% HH HH TransTrans-3--3- hepteneheptene
- 23. Equilibrates between cis and trans (more stable) LipshutzLipshutz Mechanism:Mechanism: HolidayHoliday Na dissolves in liquid ammonia, makes “solvated” electronsNa dissolves in liquid ammonia, makes “solvated” electrons
- 24. 2. Electrophilic additions.2. Electrophilic additions. Like alkenes.Like alkenes. a. HX:a. HX: RRRR ++ HH++ CC CC RR RR HH ++ XX -- Anti toAnti to HH;; pushes Rpushes R transtrans CC CC RR HH RR XX HH++ MarkovnikovMarkovnikov CC RR XX RCRCHH22 XX -- RCRCHH22CCXX22RR Geminal dihalideGeminal dihalide CHCHRCRC HXHX CC CC XX RR HH HH HXHX RCXRCX22CHCH33 Markovnikov twiceMarkovnikov twice spsp ++ spsp 22 Internal alkynesInternal alkynes Resonance with XResonance with X
- 25. δ 13 C = 202.4 ppm ++ ν = 1987 cm -1~~ 1.22 Å Linear!Linear! Angew. Chem.Angew. Chem. 20042004, 43, 43, 1543., 1543.
- 26. Examples:Examples: HBrHBr BrBr BrBr HIHI II II II II++ HClHCl ClCl ClCl Note:Note: BrBr22 BrBr BrBr NaNHNaNH22 NHNH33 HBrHBr BrBr BrBr VicinalVicinal GeminalGeminal
- 27. b. Xb. X22:: Anti additionAnti addition, as for alkenes, as for alkenes CHCH33 BrBr22 BrBr CHCH33 BrBr BrBr22 BrBr BrBr BrBr BrBr c.c. Cat.Cat. HgSOHgSO44, H, H22O hydrationO hydration, Markovnikov, Markovnikov CRCRRCRC Cat. HgSOCat. HgSO44 HH22OO CC CC HH RR OHOH RR TautomerizationTautomerization OO HH++ oror OHOH catalyzedcatalyzed -- UnstableUnstable RCHRCH22CRCR No NaBH4 needed
- 28. OO RR Mechanism of tautomerizationMechanism of tautomerization HH++ :: CC OHOH RCHRCH22 ++ RR CC OO RCHRCH22 HH++ RCHRCH22CRCR OO --HH++ OHOH :: RCHRCH22CRCR OO ++HH++ CC CC HH RR OO RR CC CC HH RR RR-- -- -- CC CC HH RR OOHH RR HH++ oror OHOH catalyzedcatalyzed --
- 29. CHCHRCRC HgSOHgSO44 HH22OO cat.cat. CC HOHO RR CHCH22 RCCHRCCH33 OO Methyl ketoneMethyl ketone d. Radical HBr:d. Radical HBr: Anti-Markovnikov additionAnti-Markovnikov addition HBrHBr ROORROOR BrBr BrBr HBrHBr -Br-Br BrBr BrBr HH HH MixturesMixtures ++
- 30. HaloalkenesHaloalkenes No SNo SNN2:2: No SNo SNN1:1:
- 31. But:But: And, metal catalysts couple alkenyl halides to alkenes in the Heck reaction:
- 33. A variant with alkynes: Sonogashira reactionA variant with alkynes: Sonogashira reaction CC CC RR HH RR XX R’C CHR’C CH++ CC CC RR HH RR R’R’
- 34. BBRR22 e. Hydroboration-Oxidatione. Hydroboration-Oxidation Use RUse R22BH (R =BH (R = bulkybulky group) togroup) to protectprotect alkenylborane: R =alkenylborane: R = cyclohexylcyclohexyl CHCHRCRC ++ B-HB-H 22 CC CC RR HH HH HH22OO22,, -- OHOH OHOH CC CC RR HH HH TautomerizationTautomerization RCHRCH22CHCH OO Aldehyde !Aldehyde ! Steric controlSteric control
- 35. 1.1. RR22BHBH 2.2. HH22OO22,,-- OHOH 1.1. HBRHBR22 2.2. HH22OO22,,-- OHOH Therefore:Therefore: HH OO butbut HgSOHgSO44 HH22OO cat.cat. OO RRRR RCCHRCCH22RR OO R = R : MixturesR = R : Mixtures
Editor's Notes
- 1:55 Billy Holiday Swing Brother Swing