F.Sc. Part 1 Chemistry Paper Faisalabad Board 2011 (Malik Xufyan)
•
0 likes•48 views
F.Sc. Part 1 Chemistry Paper Faisalabad Board 2011 (Malik Xufyan)
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Definitions and MCQs of Ninth class chemistry (solution and suspension)

Definitions and MCQs of Ninth class chemistry (solution and suspension)
Double replacement reactions with net ionic and spectators

Double replacement reactions with net ionic and spectators
SHS STEM General Chemistry MCT 17. Electrochemistry

SHS STEM General Chemistry MCT 17. Electrochemistry
First Year Chemistry_Full Book Exercise Mcqs Solved

First Year Chemistry_Full Book Exercise Mcqs Solved
MCAT CHEMISTRY SOLVED PAST PAPERS (2008-2016) - Malik Xufyan

MCAT CHEMISTRY SOLVED PAST PAPERS (2008-2016) - Malik Xufyan
SHS STEM General Chemistry MCT 9. Organic Compounds

SHS STEM General Chemistry MCT 9. Organic Compounds
SHS STEM General Chemistry MCT 1. Matter and Its Properties

SHS STEM General Chemistry MCT 1. Matter and Its Properties
Similar to F.Sc. Part 1 Chemistry Paper Faisalabad Board 2011 (Malik Xufyan)
Similar to F.Sc. Part 1 Chemistry Paper Faisalabad Board 2011 (Malik Xufyan) (20)
F.Sc. Part 1 Paper Faisalabad Board 2008 (Malik Xufyan)

F.Sc. Part 1 Paper Faisalabad Board 2008 (Malik Xufyan)
F.Sc. Part 1 Paper Faisalabad Board 2007 (Malik Xufyan)

F.Sc. Part 1 Paper Faisalabad Board 2007 (Malik Xufyan)
F.Sc. Part 1 Chemistry Paper Faisalabad Board 2010 (Malik Xufyan)

F.Sc. Part 1 Chemistry Paper Faisalabad Board 2010 (Malik Xufyan)
Cbse class 12 chemistry sample paper 05 (for 2013)

Cbse class 12 chemistry sample paper 05 (for 2013)
ORGANIC CHEMISTRY QUESTIONS FOR ADVANCED LEVEL STUDENTS

ORGANIC CHEMISTRY QUESTIONS FOR ADVANCED LEVEL STUDENTS
Tenth class-state syllabus-model paper-em-ts-physics

Tenth class-state syllabus-model paper-em-ts-physics
More from Malik Xufyan
More from Malik Xufyan (20)
KINETICS AND THERMODYNAMICS FO ENMES BY MALIK SUFYAN

KINETICS AND THERMODYNAMICS FO ENMES BY MALIK SUFYAN
Nuclease enzyme kinetics and thermodynamics by Malik Xufyan

Nuclease enzyme kinetics and thermodynamics by Malik Xufyan
Freelancing, Freelancing Skills, Freelancing Platforms 1 - Malik Xufyan

Freelancing, Freelancing Skills, Freelancing Platforms 1 - Malik Xufyan
Freelancing, Freelancing Skills, Freelancing Platforms - Malik Xufyan

Freelancing, Freelancing Skills, Freelancing Platforms - Malik Xufyan
The Contribution of Muslim Scientists in the Field of Chemistry

The Contribution of Muslim Scientists in the Field of Chemistry
PPSC Chemistry Lecturer Preparation (Test # 24)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 24)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 23)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 23)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 22)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 22)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 21)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 21)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 20)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 20)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 19)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 19)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 17)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 17)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 16)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 16)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 15)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 15)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 14)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 14)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 13)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 13)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 12)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 12)- Malik Xufyan
PPSC Chemistry Lecturer Preparation (Test # 11)- Malik Xufyan

PPSC Chemistry Lecturer Preparation (Test # 11)- Malik Xufyan
Recently uploaded
Mehran University Newsletter is a Quarterly Publication from Public Relations OfficeMehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
https://app.box.com/s/7hlvjxjalkrik7fb082xx3jk7xd7liz3TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Recently uploaded (20)
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
On National Teacher Day, meet the 2024-25 Kenan Fellows

On National Teacher Day, meet the 2024-25 Kenan Fellows
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
Unit 3 Emotional Intelligence and Spiritual Intelligence.pdf

Unit 3 Emotional Intelligence and Spiritual Intelligence.pdf
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Fostering Friendships - Enhancing Social Bonds in the Classroom

Fostering Friendships - Enhancing Social Bonds in the Classroom
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
Plant propagation: Sexual and Asexual propapagation.pptx

Plant propagation: Sexual and Asexual propapagation.pptx
ICT Role in 21st Century Education & its Challenges.pptx

ICT Role in 21st Century Education & its Challenges.pptx
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
F.Sc. Part 1 Chemistry Paper Faisalabad Board 2011 (Malik Xufyan)
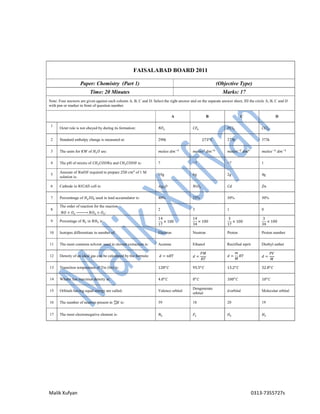
- 1. Malik Xufyan 0313-7355727s FAISALABAD BOARD 2011 Paper: Chemistry (Part 1) (Objective Type) Time: 20 Minutes Marks: 17 Note: Four answers are given against each column A, B, C and D. Select the right answer and on the separate answer sheet, fill the circle A, B, C and D with pen or marker in front of question number. A B C D 1 Octet role is not obeyed by during its formation: 𝑁𝐹 𝐶𝐹 𝑃𝐶𝑙 𝐶𝐶𝑙 2 Standard enthalpy change is measured at: 298k 273 𝐶 273k 373k 3 The units for 𝐾𝑊 of 𝐻 𝑂 are: 𝑚𝑜𝑙𝑒𝑠 𝑑𝑚 𝑚𝑜𝑙𝑒𝑠 𝑑𝑚 𝑚𝑜𝑙𝑒𝑠 𝑑𝑚 𝑚𝑜𝑙𝑒𝑠 𝑑𝑚 4 The pH of mixtre of 𝐶𝐻 𝐶𝑂𝑂𝑁𝑎 and 𝐶𝐻 𝐶𝑂𝑂𝐻 is: 7 >7 <7 1 5 Amount of 𝑁𝑎𝑂𝐻 required to prepare 250 𝑐𝑚 of 1 M solution is: 10g 6g 2g 4g 6 Cathode in 𝑁𝐼𝐶𝐴𝐷 cell is: 𝐴𝑔 𝑂 𝑁𝑖𝑂 𝐶𝑑 𝑍𝑛 7 Percentnage of 𝐻 𝑆𝑂 used in lead accumulator is: 40% 25% 30% 50% 8 The order of reaction for the reaction 𝑁𝑂 + 𝑂 ⎯⎯⎯⎯⎯ 𝑁𝑂 + 𝑂 : 2 3 1 0 9 Percentage of 𝑁 in 𝑁𝐻 is: 14 17 × 100 14 34 × 100 3 17 × 100 3 34 × 100 10 Isotopes differentiate in number of: Electron Neutron Proton Proton number 11 The most common solvent used in slovent extraction is: Acetone Ethanol Rectified siprit Diethyl eather 12 Density of an ideal gas can be calculated by the formula: 𝑑 = 𝑛𝑅𝑇 𝑑 = 𝑃𝑀 𝑅𝑇 𝑑 = 𝑚 𝑀 𝑅𝑇 𝑑 = 𝑃𝑉 𝑀 13 Transition temperature of Tin (Sn) is: 128 𝐶 95.5 𝐶 13.2 𝐶 32.8 𝐶 14 Whater has maximus density at: 4.0 𝐶 0 𝐶 100 𝐶 10 𝐶 15 Orbitals having equal energy are called: Valence orbital Dengenerate orbital d-orbital Molecular orbital 16 The number of neutron present in 𝐾 is: 39 18 20 19 17 The most electronegative element is: 𝑁 𝐹 𝑂 𝐻
- 2. Malik Xufyan 0313-7355727s SECTION-1 2. Attempt any 22 short questions: ( 44) (i) List steps involved to identify a limiting reactant? (ii) Explain relative abundant of isotopes with suitable example? (iii) A limiting reactant controls the amount of reactant consumed in a chemical reaction. Comment the statement? (iv) Define Rf value and why it has no unit? (v) Iodine is more soluble in water in pressure of KI. Discuss. (vi) List four postulates of kinetic theory? (vii) Define Dalton’s law of partial pressure. Give mathematical expression? (viii) Gases show non-ideal behavior at low temperature and high pressure. Justify the statement. (ix) Ice floats on 𝐻 𝑂. Give reason? (x) Explain the working of pressure cooker? (xi) Describe crystallographic elements? (xii) Graphite is conductor but diamond is insulator. Give reason? (xiii) Write electronic configuration foe an element with atomic number Z=29. (xiv) Describe Stark and Zeeman Effect? (xv) Complete: 𝐻𝑒 + 𝐵𝑒 ⎯⎯⎯⎯⎯⎯ 𝐻 + ______ (xvi) Calculate the mass of an electron when 𝑒 𝑚⁄ = 1.7588 × 10 𝐶𝑜𝑙𝑢𝑚𝑏𝑠 𝑘𝑔 ? (xvii) How will you prepare 10% 𝑤 𝑣⁄ urea solute in water? (xviii) The concentration in terms of molality is independent of temperature but molarity depends upon temperature. Why? (xix) The freezed points are depressed due to the pressure of solutes. Give reasons. (xx) Calculate the oxidation number of N in 𝑁𝐻𝑂 and 𝑁𝑂 ? (xxi) Na and K can displace hydrogen from acids but Pt, Pd and Cu cannot. Comment on it. (xxii) A porous plate or a salt bridge is not required in lead storage cell. Give reason? (xxiii) How the enzymes act as catalysts? (xxiv) A particular catalyst is suitable for a particular chemical reaction. Justify it. (xxv) What is coordinate covalent bond? Give example? (xxvi) Define electronegativity. Give its trend in periodic table? (xxvii) What is the cause for the chemical change? (xxviii) Differentiate between internal energy and enthalpy? (xxix) Differentiate between spontaneous and non – spontaneous reaction? (xxx) Solubility of glucose increase in water by heating. Give reason? (xxxi) What factors influence the electron affinity? (xxxii) What will the effect of volume change on the following system at equilibrium state: (a) PCl ( ) ⇌ PCl ( ) + Cl ( ) (b) 2SO ( ) + O ( ) ⇌ 2SO ( ) (xxxiii) How Kc predict the extent of chemical of a chemical reaction? SECTION-2 Note: Attempt any THREE questions 3. (a) Define liquid crystals and write its three uses? (b) The combustion analysis of an organic compound shows it to contain 65.44 % of carbon, 5.50% of hydrogen, 29.06% of oxygen. What is the empirical formula for the compound? If molecular mass of this compound is 110.15𝑔. 𝑚𝑜𝑙 . Calcuate the molecular formula of the compound? 4. (a) Describe the Dalton’s law of partial pressure and write its two applications? (b) Derive the formula for calculating the energy of an electron in nth orbit using Bohar’s model. 5. (a) Define the term electronegativity. Discuss its variation in the periodic table? (b) How ∆𝐻 can be determined by using bomb calorimeter? Time: 02:40 Hours (Subjective Type) Marks: 68
- 3. Malik Xufyan 0313-7355727s 6. (a) Balance the equation by oxidation number method: 𝐾 𝐶𝑟 𝑂 + 𝐻𝐶𝑙 ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯ 𝐾𝐶𝑙 + 𝐶𝑙 + 𝐻 𝑂 (b) 7. (a) Define colligative properties. Explain elevation of boiling point. (b) Define the terms: (1) Rate of reaction (2) Order of reaction
