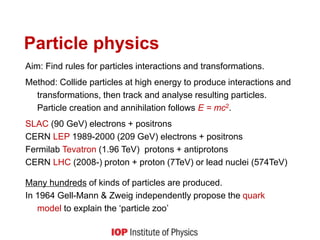
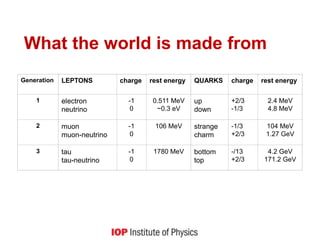
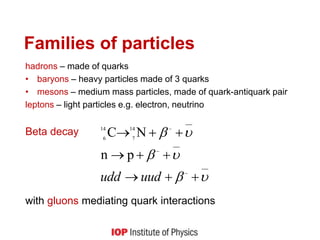
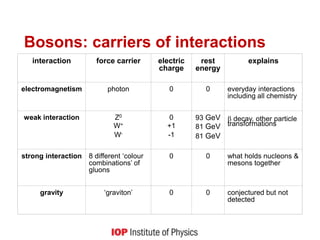
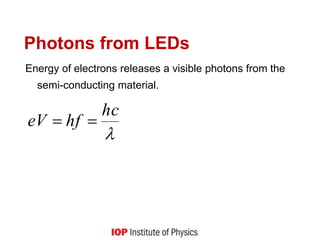
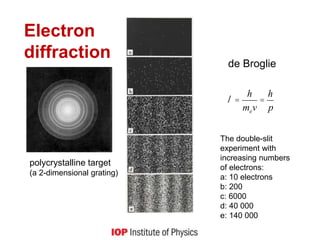
This document provides an overview of quantum phenomena and related concepts:
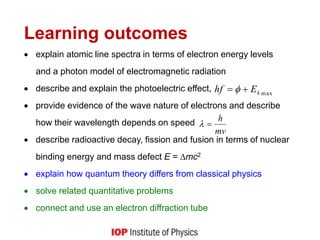
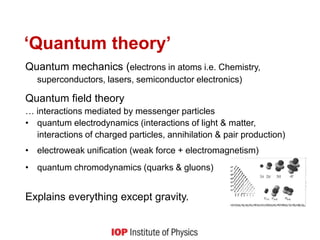
1) It explains key quantum concepts like electron energy levels, photon emission and absorption, the photoelectric effect, electron diffraction, radioactive decay, and how quantum theory differs from classical physics.
2) It discusses challenges in teaching quantum theory like its counterintuitive nature and need to link abstract ideas to observable phenomena.
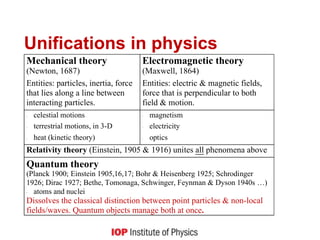
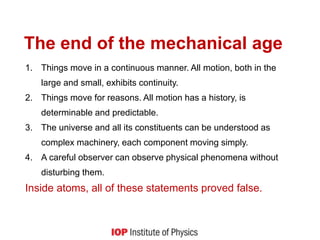
3) It provides historical context on the development of quantum theory and unifications in physics, noting how quantum theory dissolved the classical distinction between particles and fields.









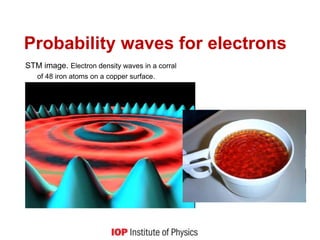
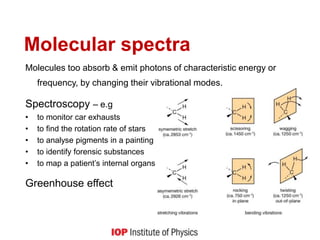
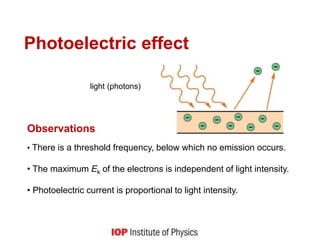

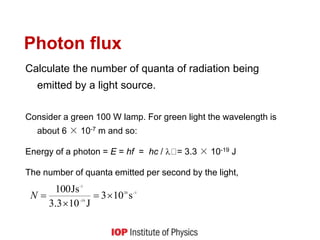
![Quantum behaviour
• not particle behaviour
• not wave behaviour
Young’s double slit with photons: Wave calculations give the
right answers for where there are bright and dark fringes. Physicists
use the wave calculation but don’t think of light simply as waves.
Phase is an intrinsic property of quantum objects, so interference
can result from path differences. [analytic tool is ‘phasor’ ]
h
E
f ](https://image.slidesharecdn.com/quantum-phenomena-220910171619-867d625e/85/Quantum-phenomena-ppt-21-320.jpg)