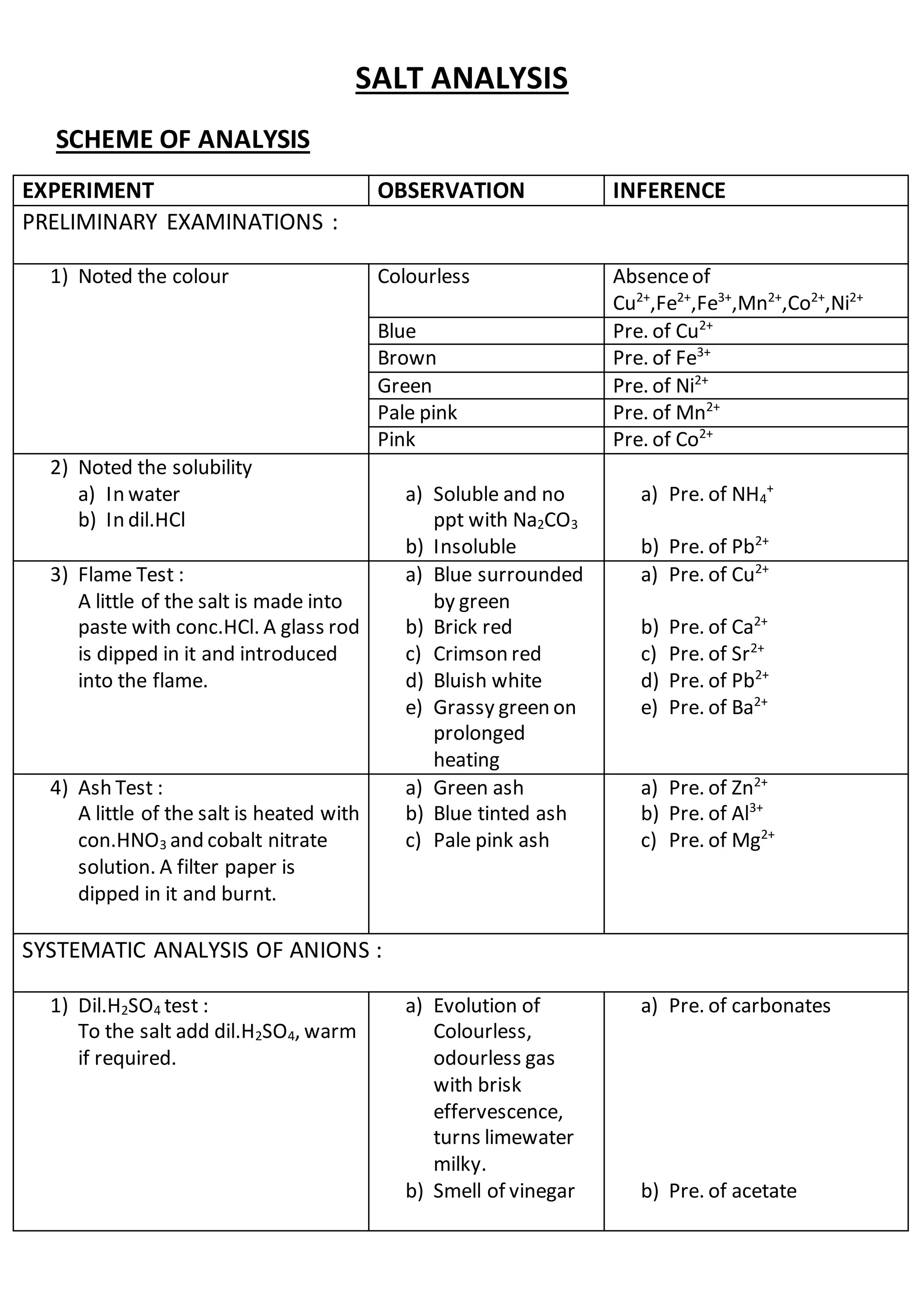
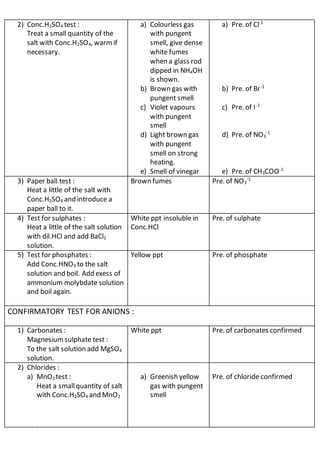
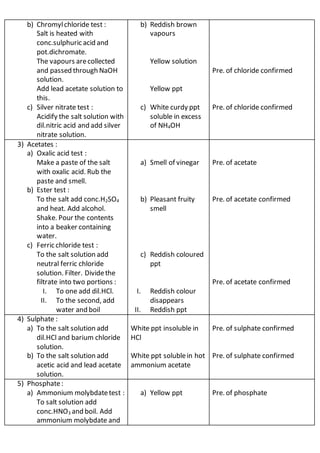
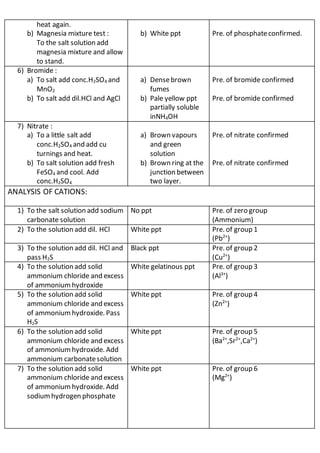
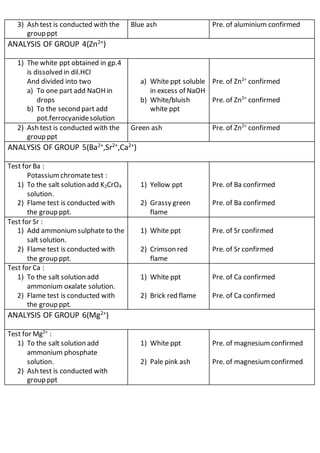
This document outlines the procedures and observations for analyzing an unknown salt sample. It describes preliminary examinations to identify possible cations present. It then details systematic analysis of anions using acid and base tests. Specific anions like carbonates, chlorides, acetates and others are tested for. The document concludes by describing analysis of cation groups through precipitation reactions and tests, identifying possibilities like ammonium, lead, copper, aluminum, zinc, barium/strontium/calcium, and magnesium. The goal is to determine the identities of the anions and cations present in the unknown salt.