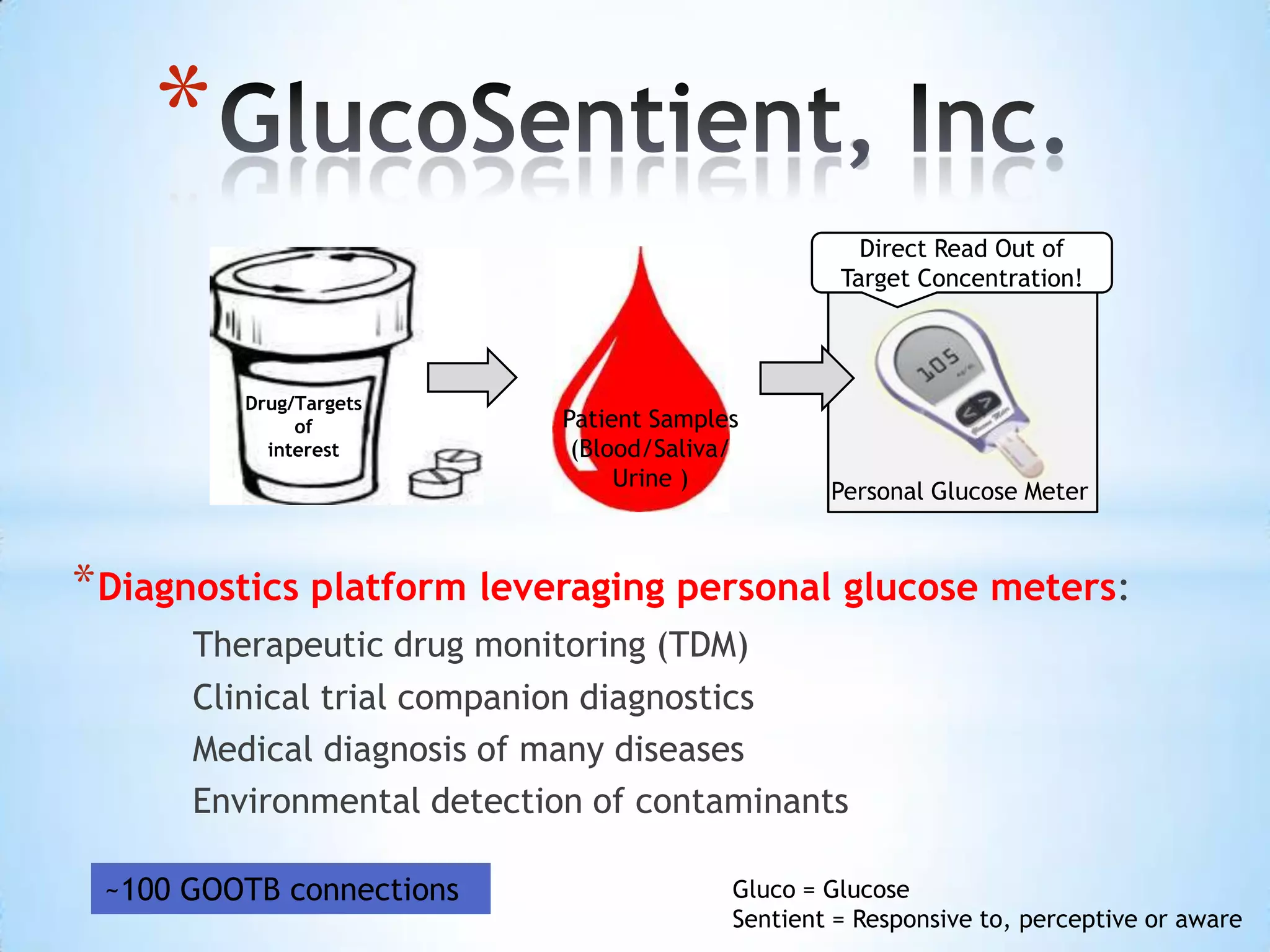
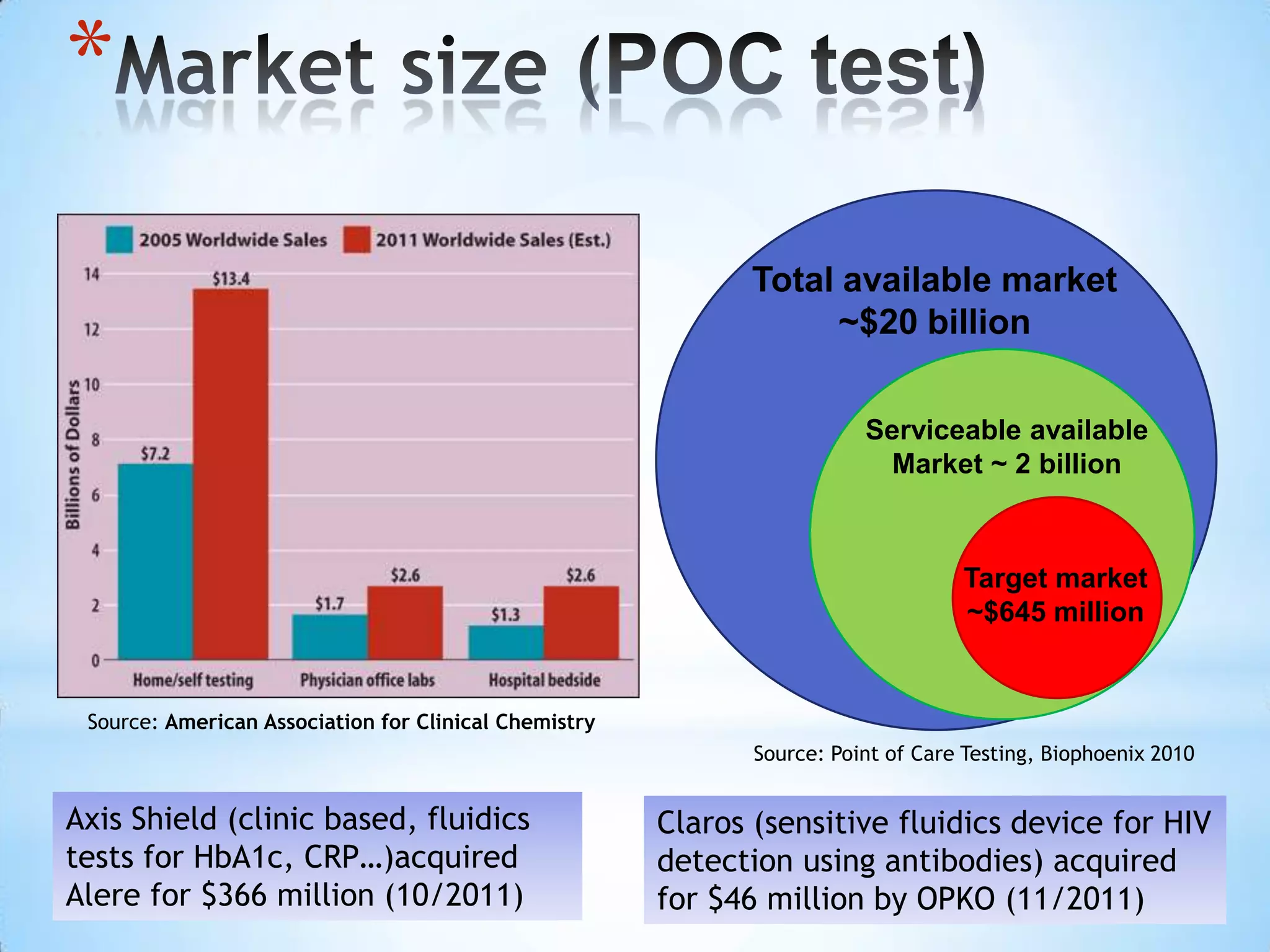
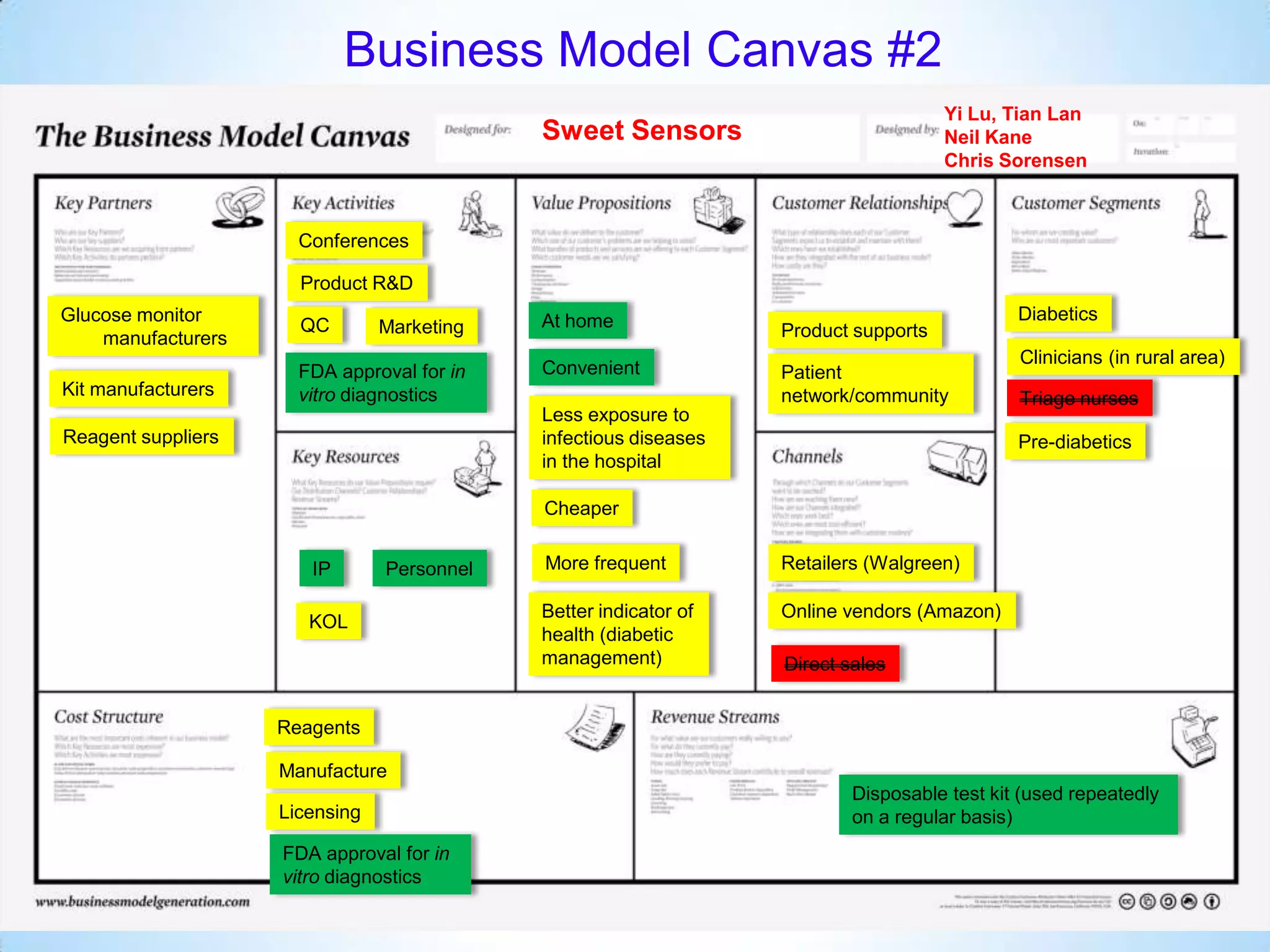
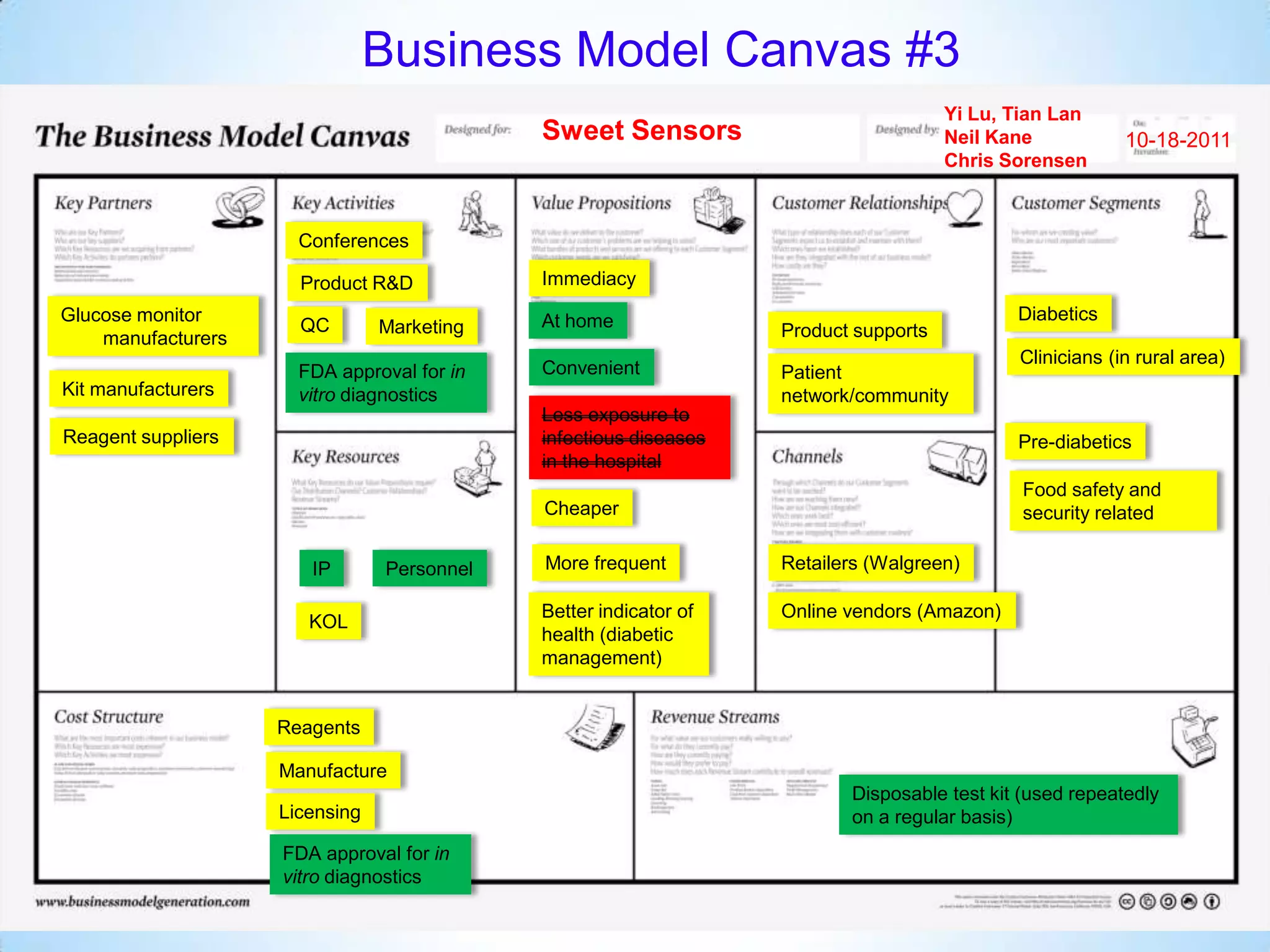
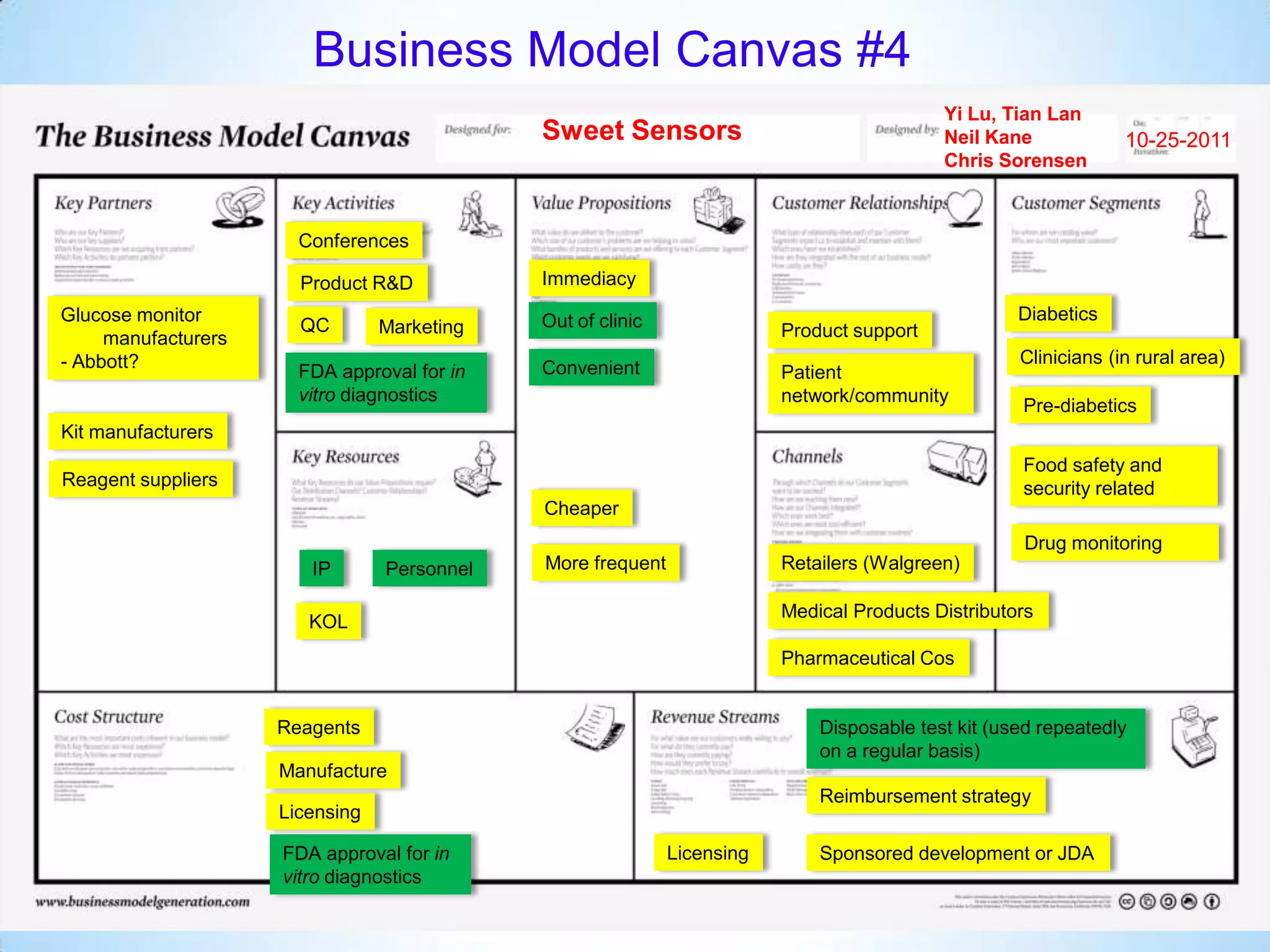
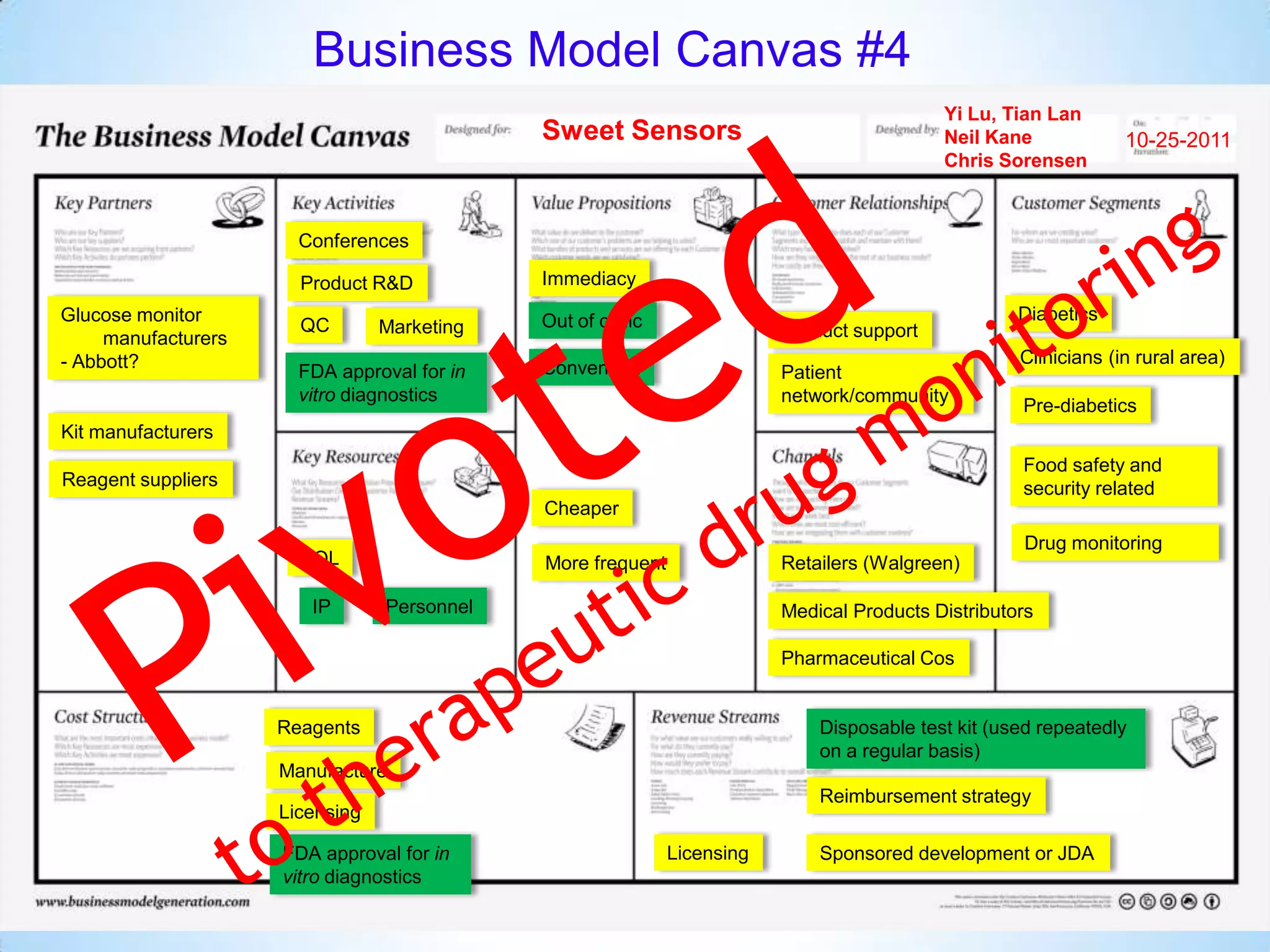
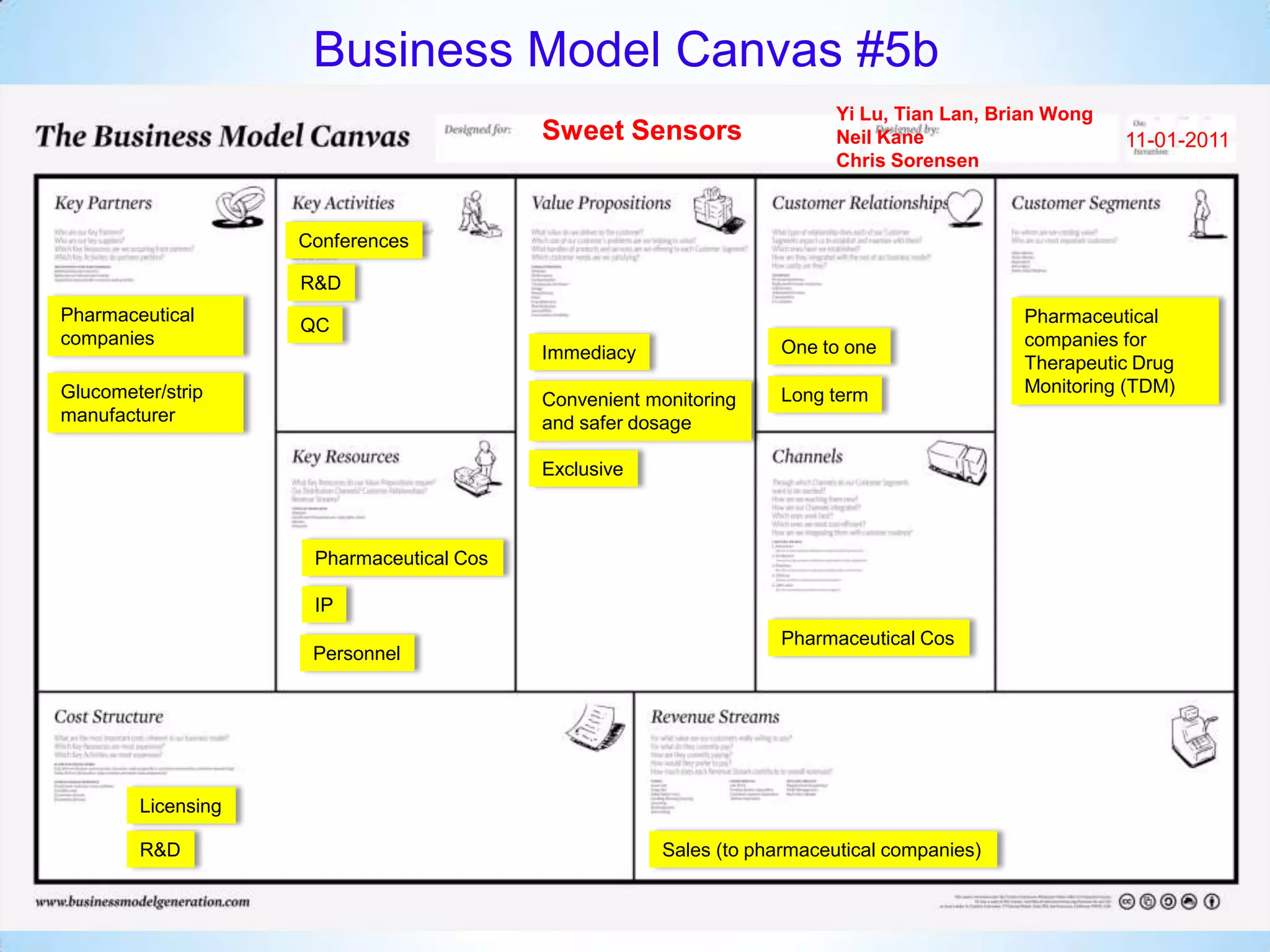
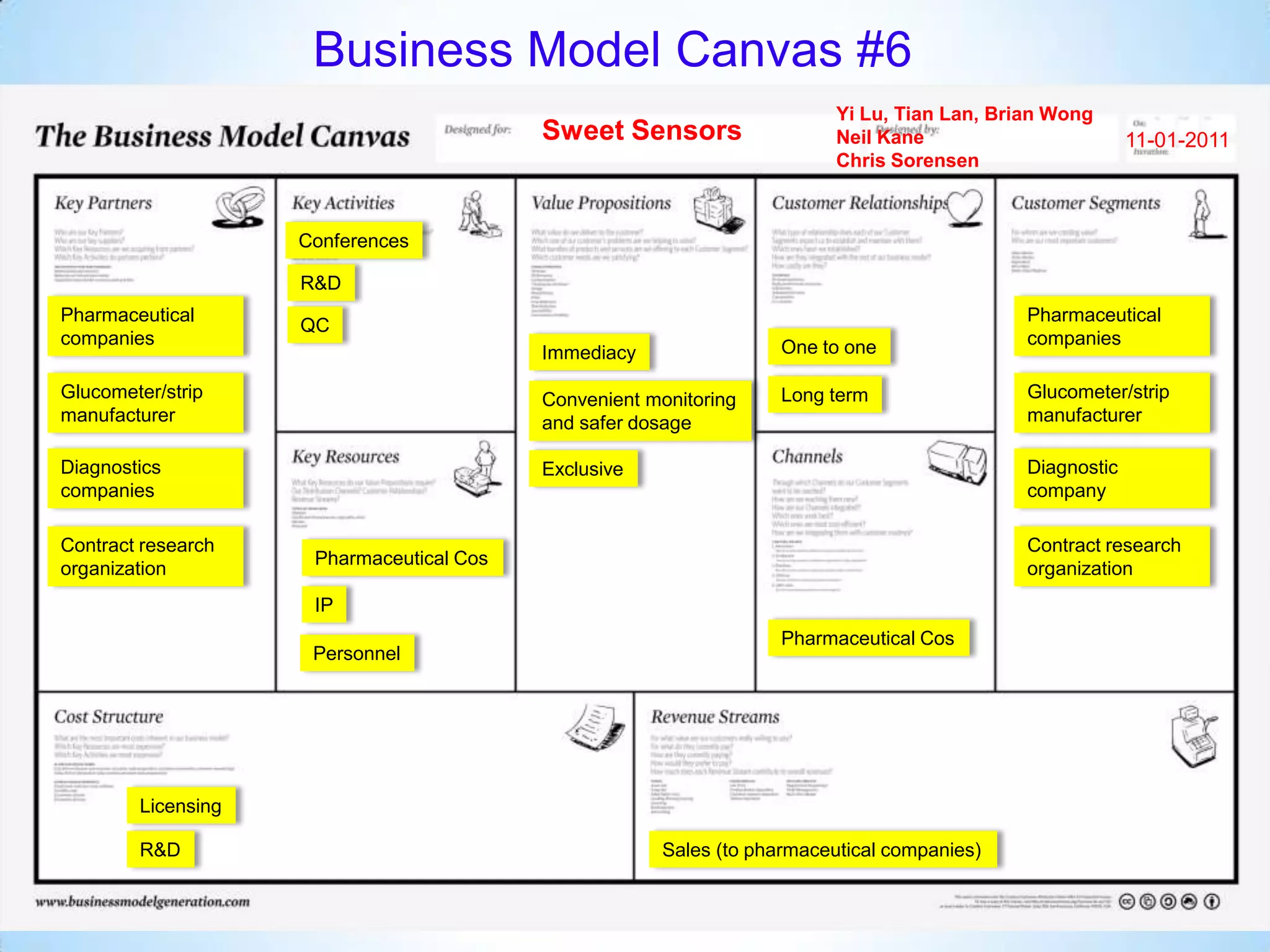
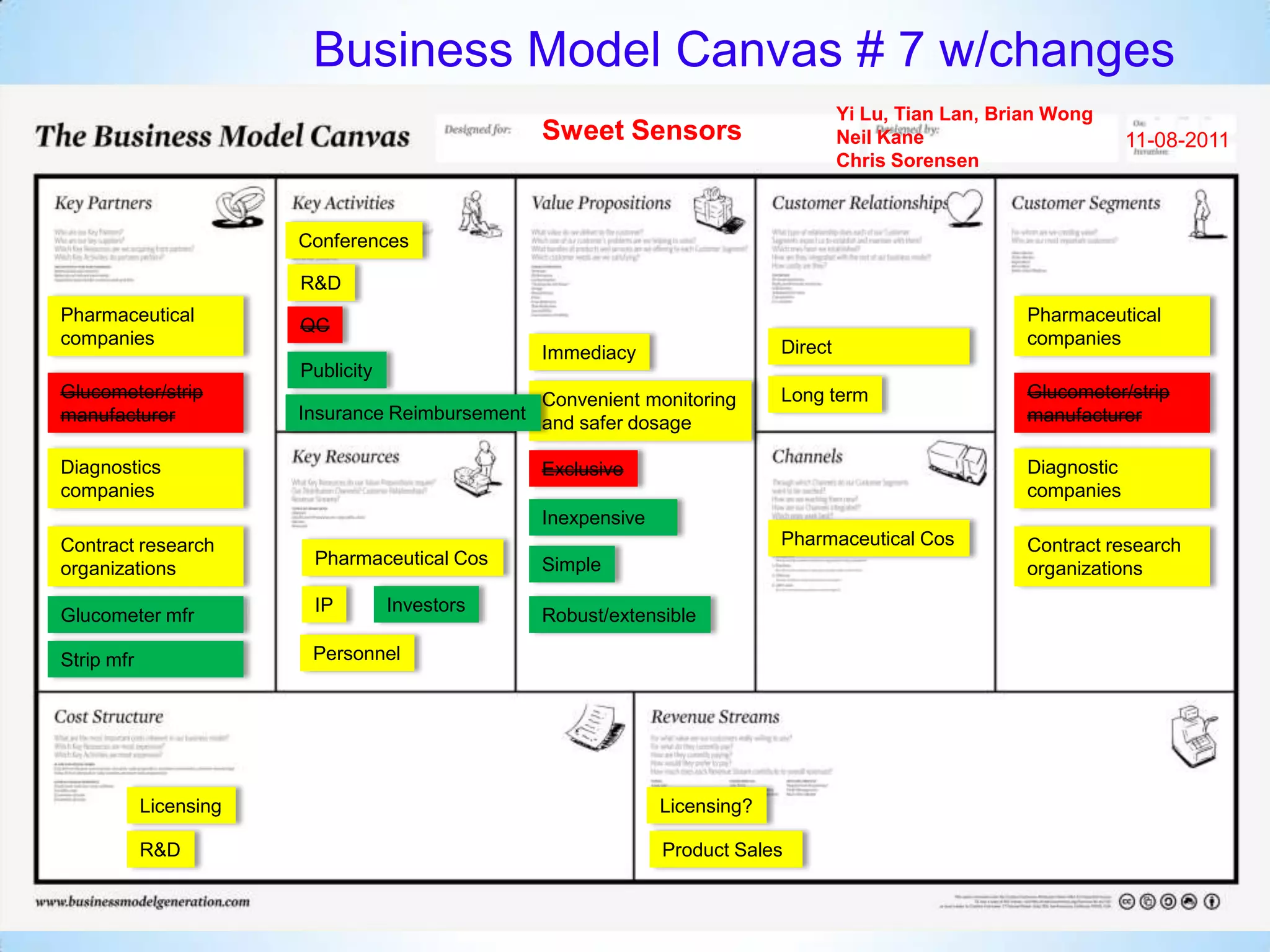
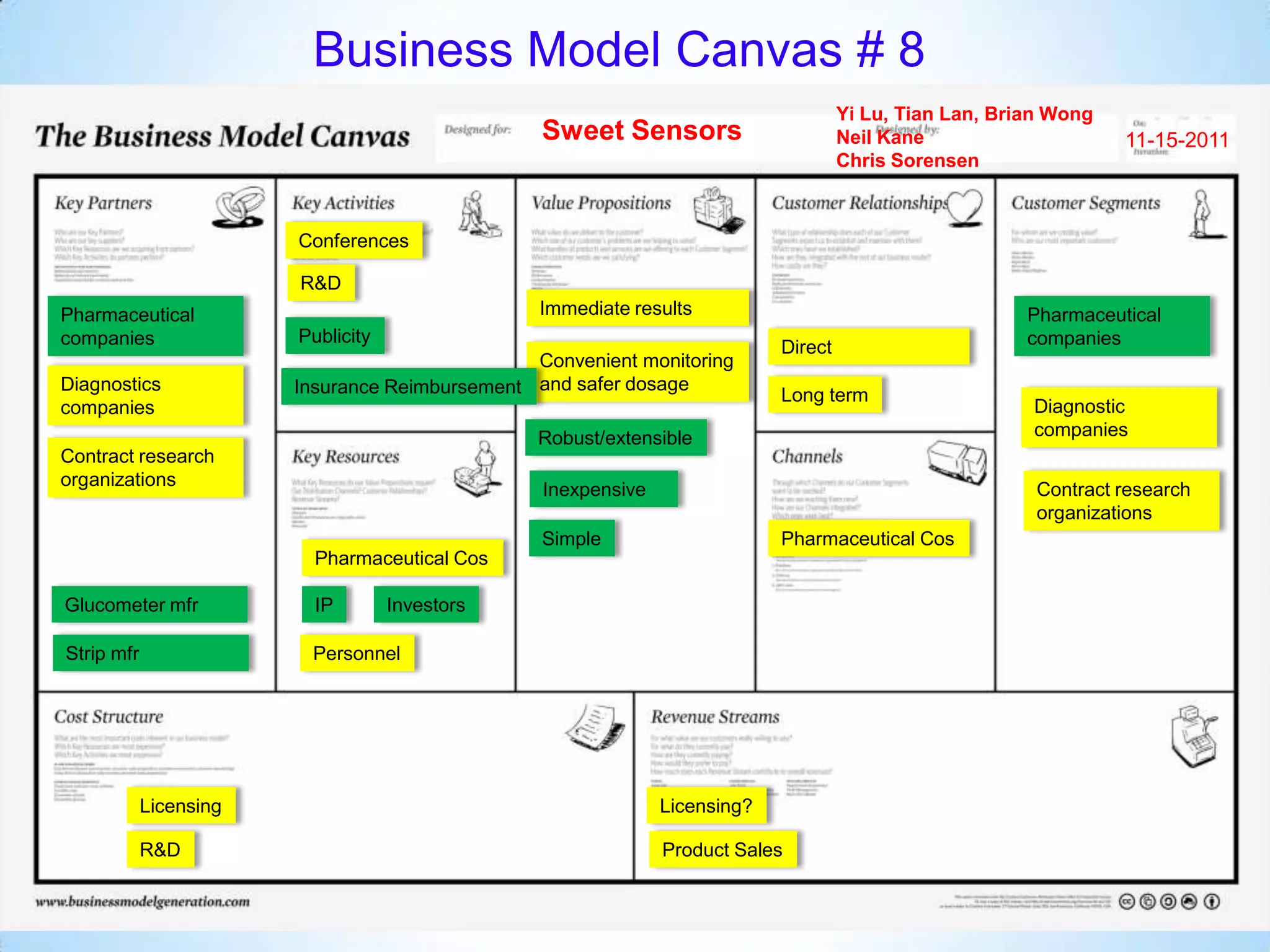
The document proposes a diagnostics platform that leverages personal glucose meters to directly read out target concentrations from patient samples. This allows for therapeutic drug monitoring, clinical trial companion diagnostics, and medical diagnosis of many diseases.
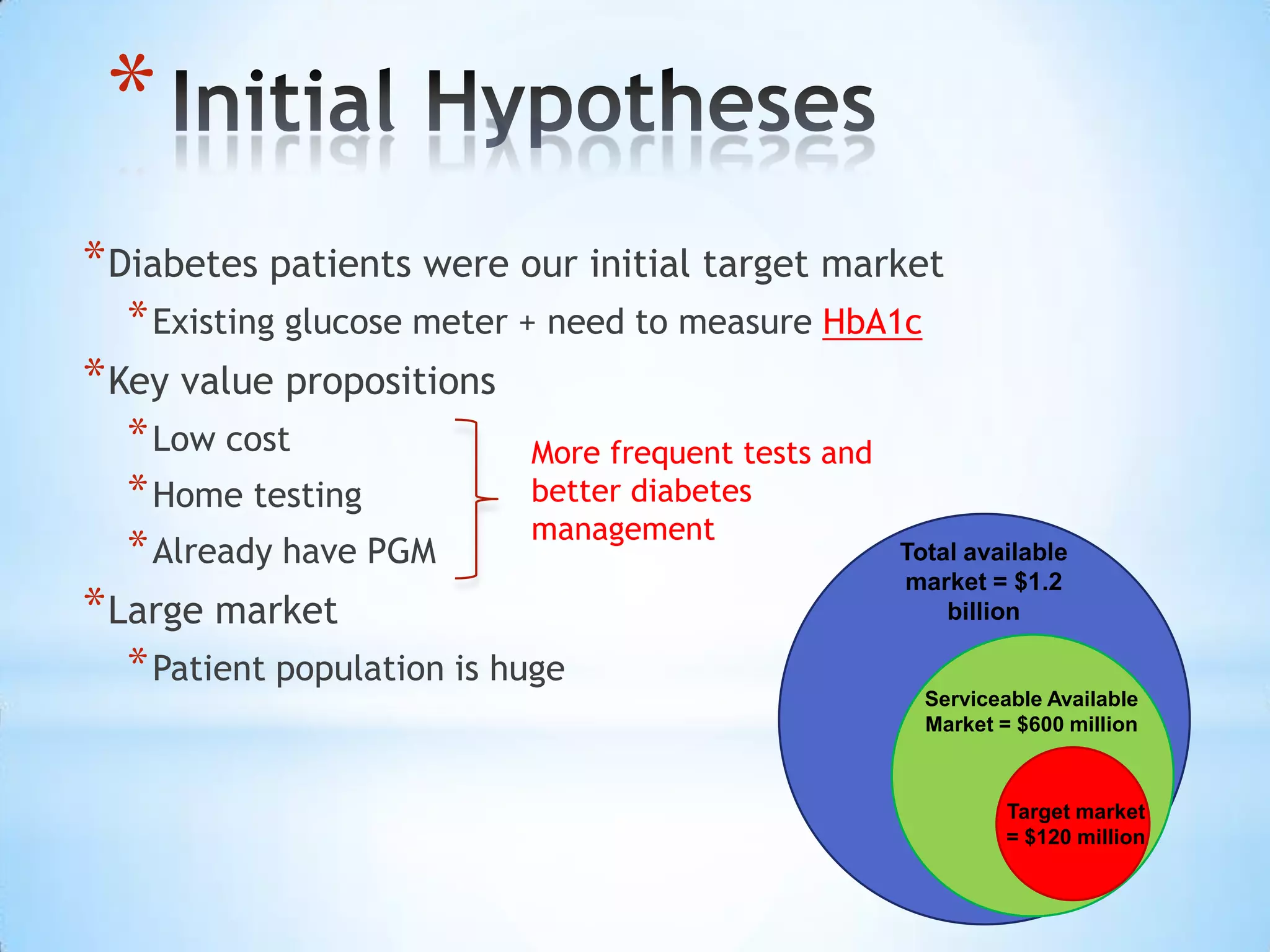
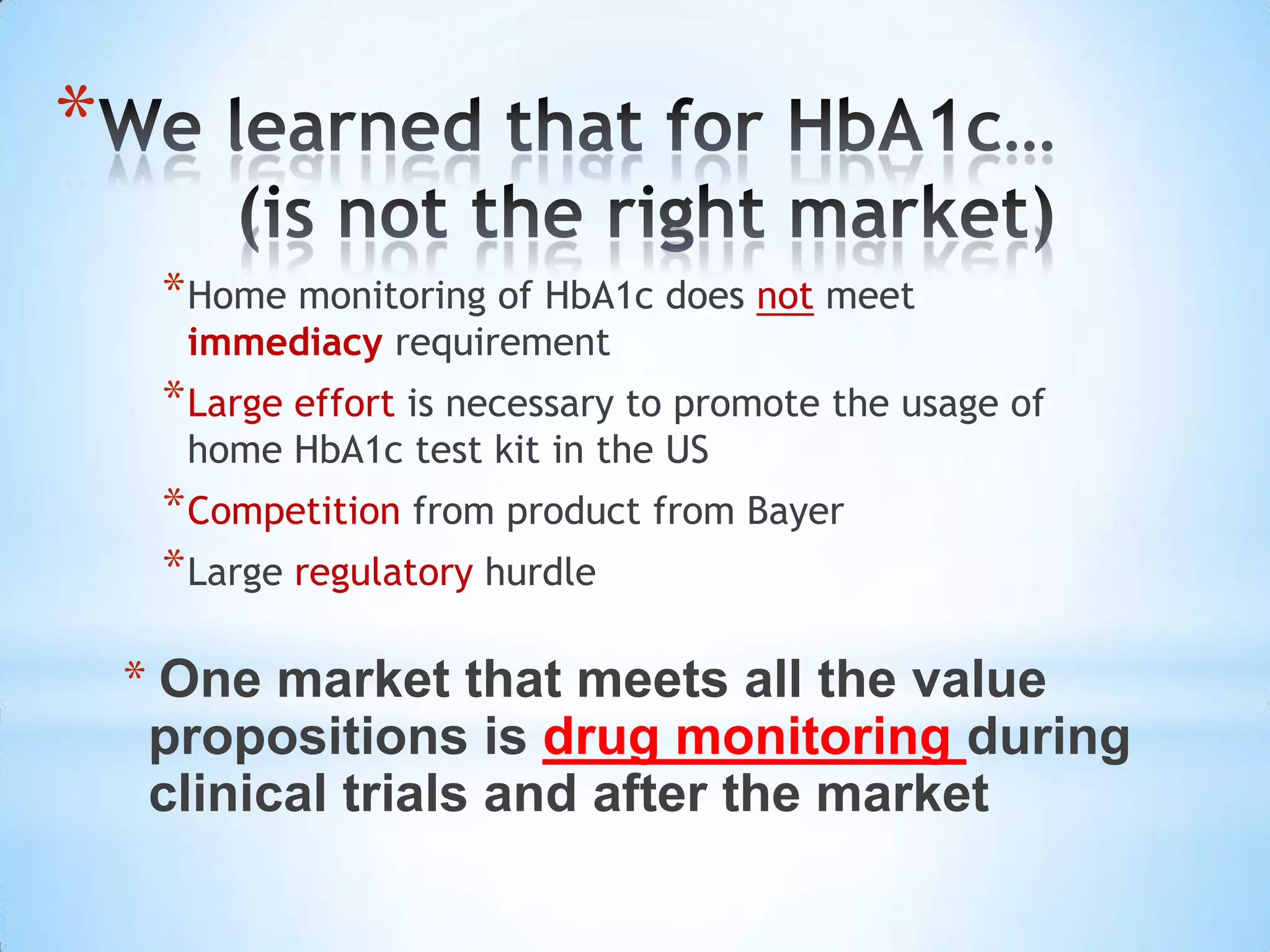
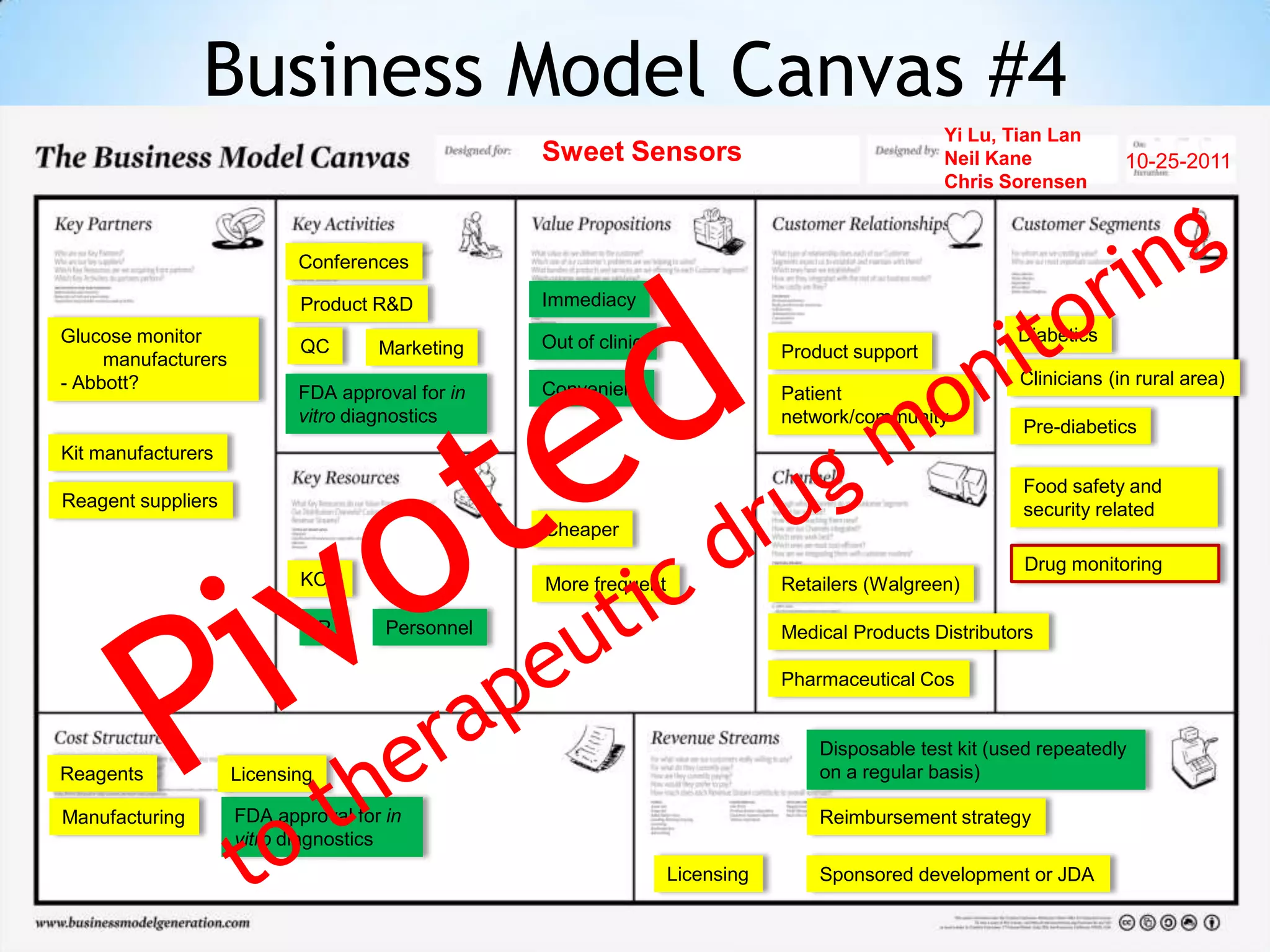
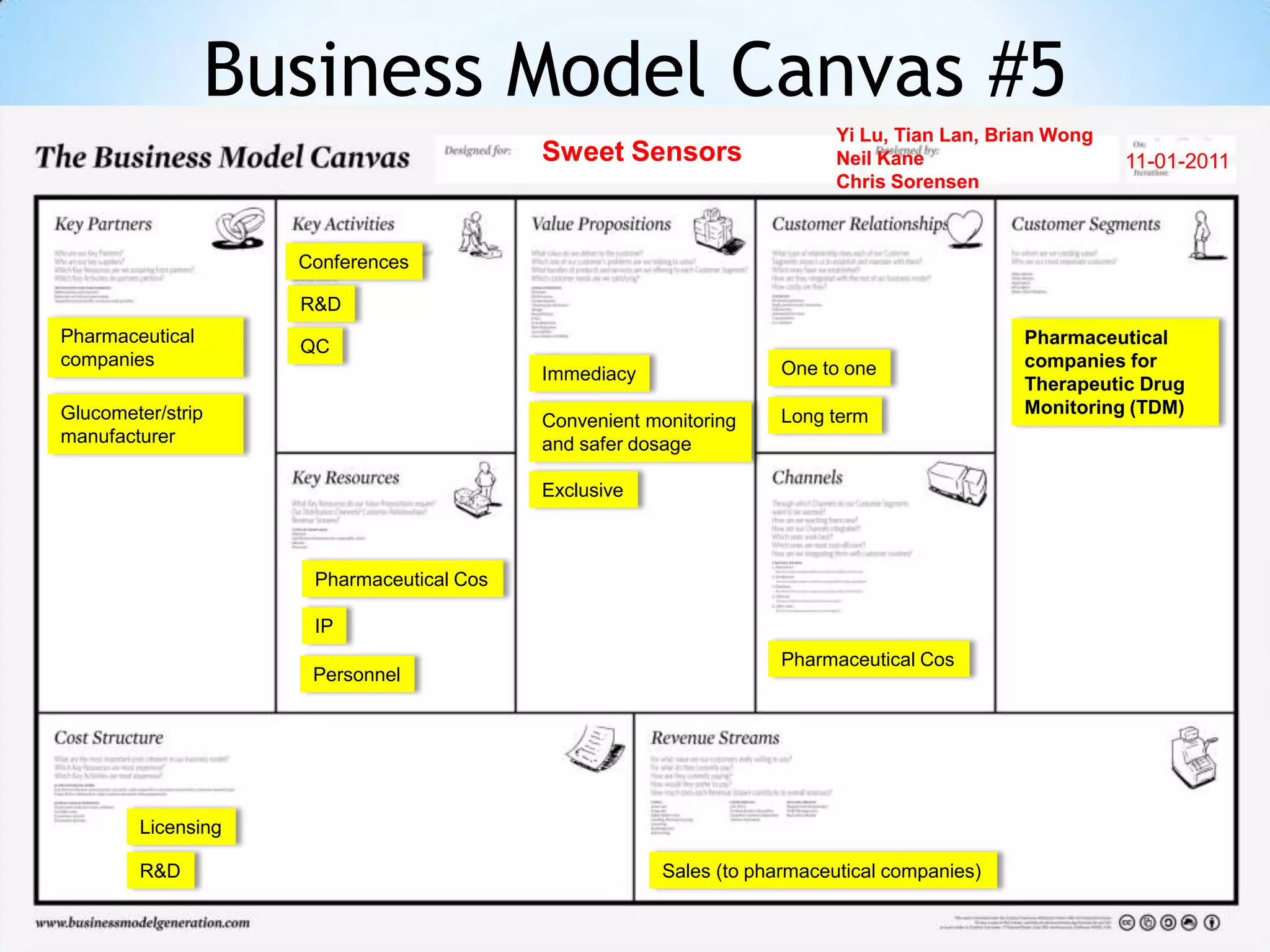
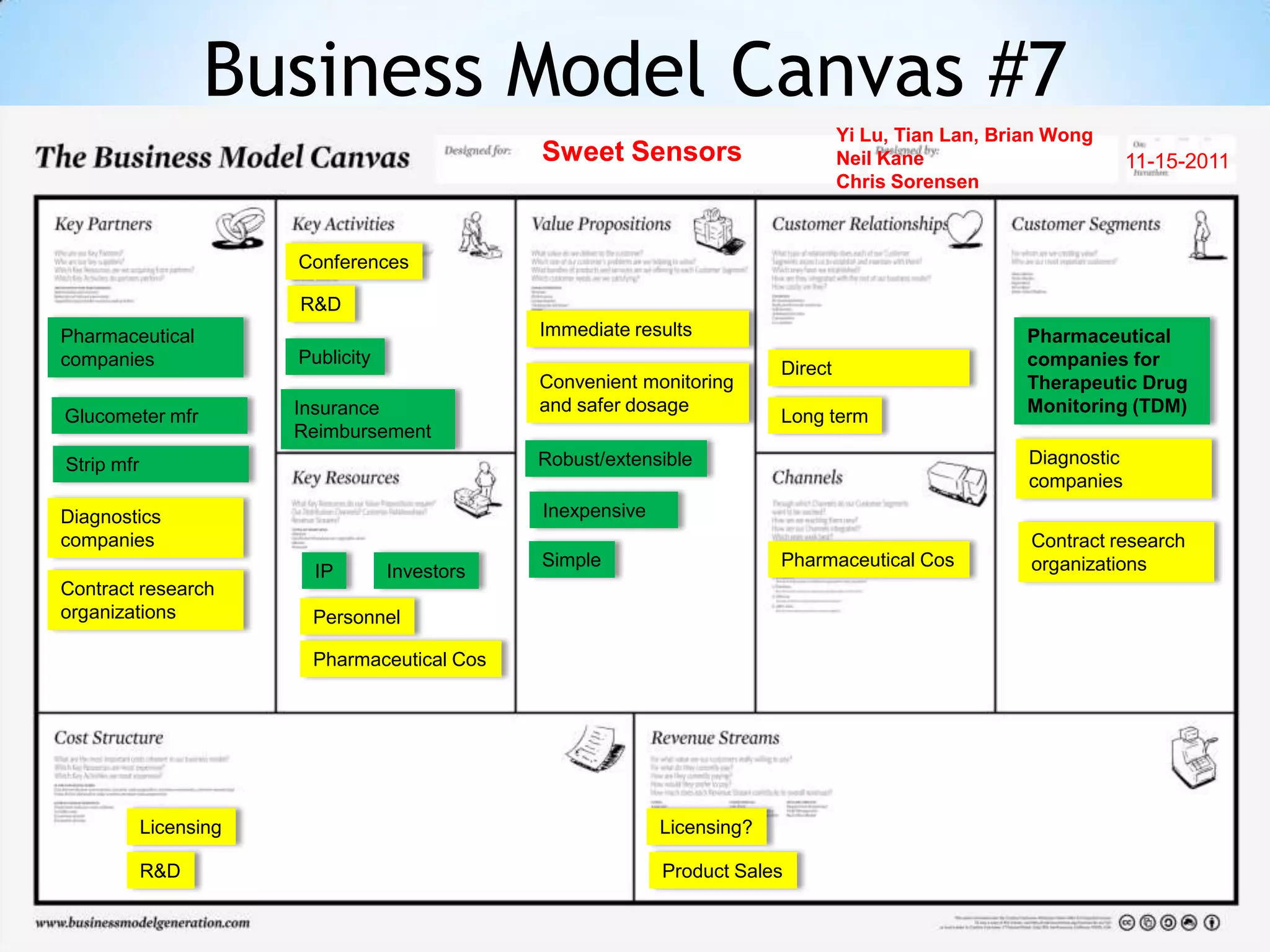
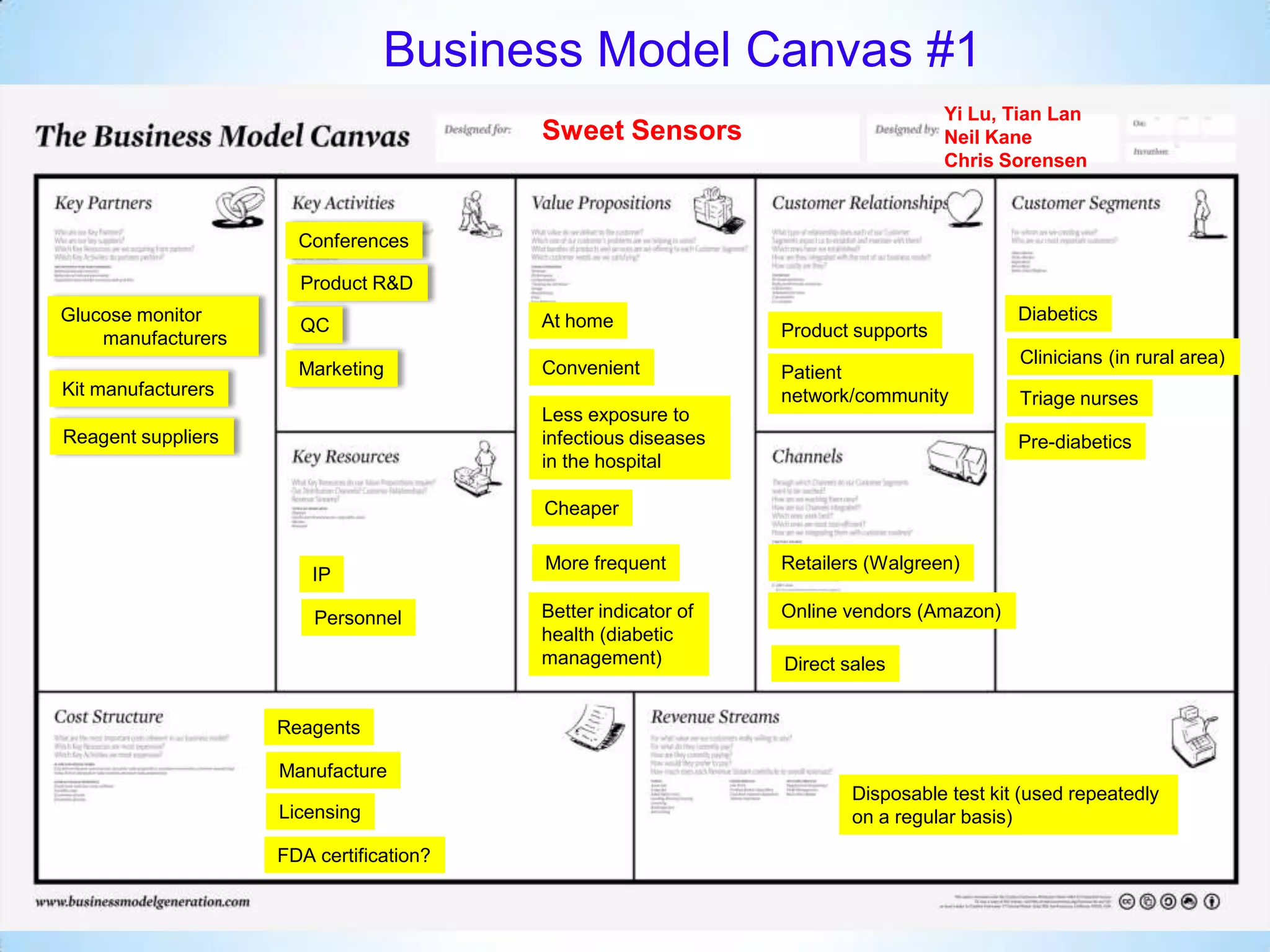
Initial targets were diabetes patients to measure HbA1c using existing glucose meters. However, drug monitoring during clinical trials and after market approval emerged as a better fit due to meeting value propositions of immediacy, low cost, convenience, and allowing for more frequent testing.
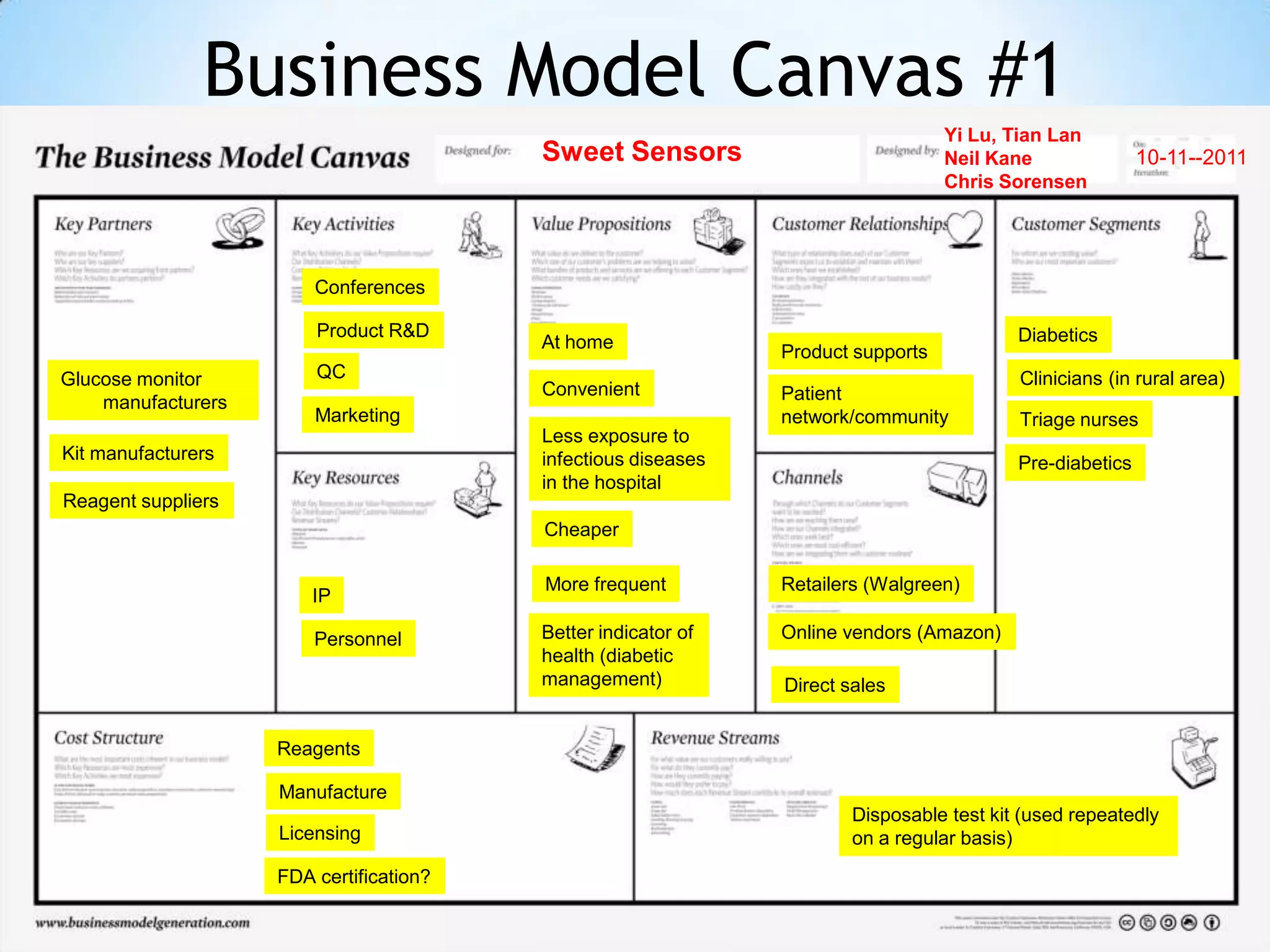
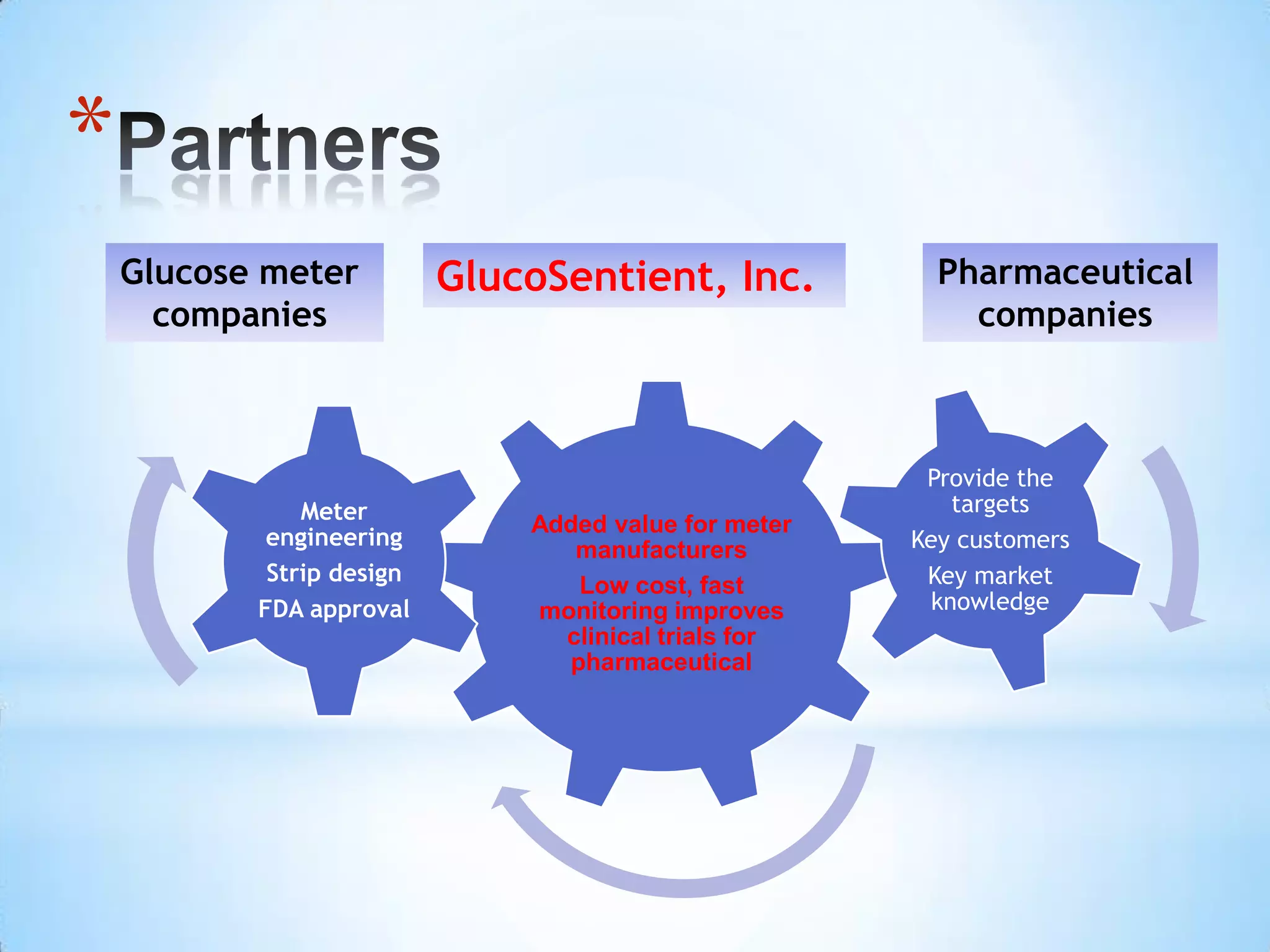
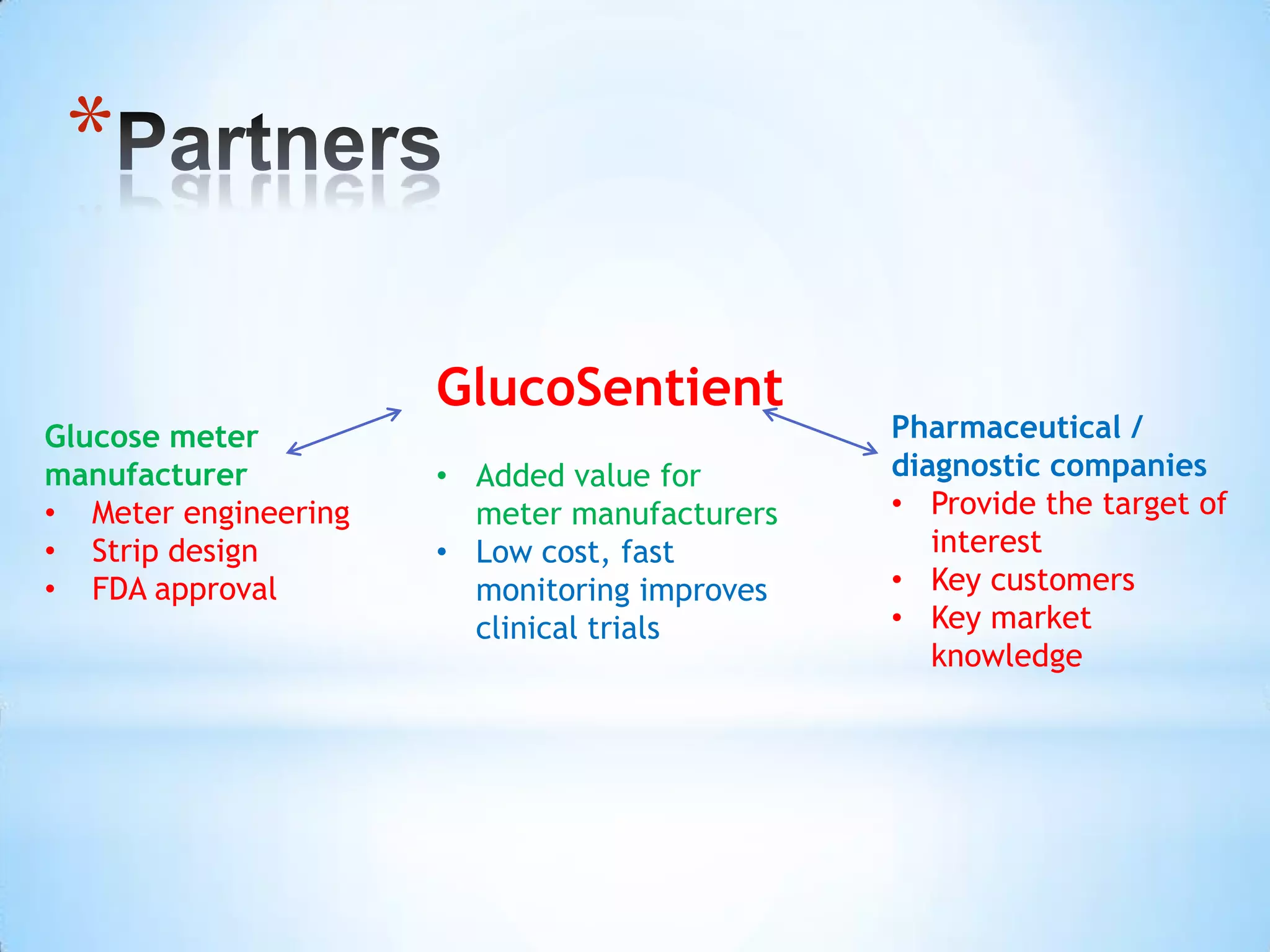
Partnering with pharmaceutical companies provides financial support and an outlet to sell final products. Glucose meter companies and pharmaceutical companies are key customers and markets due to improved drug development and clinical trials from fast, low-cost monitoring improving