Chemical equations
•Download as PPT, PDF•
4 likes•4,693 views
Report
Share
Report
Share
More Related Content
What's hot
What's hot (20)
Properties and Formation of Ionic Compounds Powerpoint

Properties and Formation of Ionic Compounds Powerpoint
Similar to Chemical equations
Similar to Chemical equations (20)
CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 1 WRI...

CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 1 WRI...
Ch8 balancingchemicalreactions-121219135210-phpapp02

Ch8 balancingchemicalreactions-121219135210-phpapp02
Balancing_Chemical_Equations_using_the_Law_of_Mass.ppt

Balancing_Chemical_Equations_using_the_Law_of_Mass.ppt
Balancing_Chemical_Equations_using_the_Law_of_Mass.ppt

Balancing_Chemical_Equations_using_the_Law_of_Mass.ppt
More from Grover Cleveland Middle School
More from Grover Cleveland Middle School (20)
Ocean acidification will-the_reef_survive-non-narrated[1]![Ocean acidification will-the_reef_survive-non-narrated[1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Ocean acidification will-the_reef_survive-non-narrated[1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Ocean acidification will-the_reef_survive-non-narrated[1]
Ocean acidification will-the_reef_survive-non-narrated[1]![Ocean acidification will-the_reef_survive-non-narrated[1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Ocean acidification will-the_reef_survive-non-narrated[1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Ocean acidification will-the_reef_survive-non-narrated[1]
Recently uploaded
Model Call Girl in Tilak Nagar Delhi reach out to us at 🔝9953056974🔝

Model Call Girl in Tilak Nagar Delhi reach out to us at 🔝9953056974🔝9953056974 Low Rate Call Girls In Saket, Delhi NCR
FINALS_OF_LEFT_ON_C'N_EL_DORADO_2024.pptx

FINALS_OF_LEFT_ON_C'N_EL_DORADO_2024.pptxConquiztadors- the Quiz Society of Sri Venkateswara College
call girls in Kamla Market (DELHI) 🔝 >༒9953330565🔝 genuine Escort Service 🔝✔️✔️

call girls in Kamla Market (DELHI) 🔝 >༒9953330565🔝 genuine Escort Service 🔝✔️✔️9953056974 Low Rate Call Girls In Saket, Delhi NCR
Recently uploaded (20)
MULTIDISCIPLINRY NATURE OF THE ENVIRONMENTAL STUDIES.pptx

MULTIDISCIPLINRY NATURE OF THE ENVIRONMENTAL STUDIES.pptx
TataKelola dan KamSiber Kecerdasan Buatan v022.pdf

TataKelola dan KamSiber Kecerdasan Buatan v022.pdf
Barangay Council for the Protection of Children (BCPC) Orientation.pptx

Barangay Council for the Protection of Children (BCPC) Orientation.pptx
4.18.24 Movement Legacies, Reflection, and Review.pptx

4.18.24 Movement Legacies, Reflection, and Review.pptx
Model Call Girl in Tilak Nagar Delhi reach out to us at 🔝9953056974🔝

Model Call Girl in Tilak Nagar Delhi reach out to us at 🔝9953056974🔝
call girls in Kamla Market (DELHI) 🔝 >༒9953330565🔝 genuine Escort Service 🔝✔️✔️

call girls in Kamla Market (DELHI) 🔝 >༒9953330565🔝 genuine Escort Service 🔝✔️✔️
Visit to a blind student's school🧑🦯🧑🦯(community medicine)

Visit to a blind student's school🧑🦯🧑🦯(community medicine)
How to do quick user assign in kanban in Odoo 17 ERP

How to do quick user assign in kanban in Odoo 17 ERP
Procuring digital preservation CAN be quick and painless with our new dynamic...

Procuring digital preservation CAN be quick and painless with our new dynamic...
Chemical equations
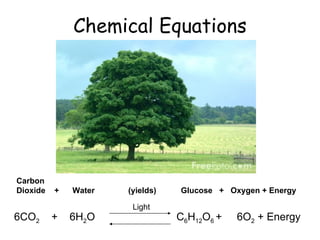
- 1. Chemical Equations 6CO 2 + 6H 2 O Carbon Dioxide + Water (yields) Glucose + Oxygen + Energy C 6 H 12 O 6 + 6O 2 + Energy Light