Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Atoms and Molecules, Ions, Isobars, Isotops etc... Chemistry PPT

Atoms and Molecules, Ions, Isobars, Isotops etc... Chemistry PPT
Viewers also liked (7)
Laws of chemical combinations, prepared by Saliha Rais

Laws of chemical combinations, prepared by Saliha Rais
Similar to Atoms & molecules
Similar to Atoms & molecules (20)
Occupational Therapy Chemical Composition of the Human Body

Occupational Therapy Chemical Composition of the Human Body
Basic Concept of Chemistry new.pptx Wajid ullah umar

Basic Concept of Chemistry new.pptx Wajid ullah umar
Recently uploaded
Making communications land - Are they received and understood as intended? webinar
Thursday 2 May 2024
A joint webinar created by the APM Enabling Change and APM People Interest Networks, this is the third of our three part series on Making Communications Land.
presented by
Ian Cribbes, Director, IMC&T Ltd
@cribbesheet
The link to the write up page and resources of this webinar:
https://www.apm.org.uk/news/making-communications-land-are-they-received-and-understood-as-intended-webinar/
Content description:
How do we ensure that what we have communicated was received and understood as we intended and how do we course correct if it has not.Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...Association for Project Management
Recently uploaded (20)
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
Seal of Good Local Governance (SGLG) 2024Final.pptx

Seal of Good Local Governance (SGLG) 2024Final.pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Micro-Scholarship, What it is, How can it help me.pdf

Micro-Scholarship, What it is, How can it help me.pdf
UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf

UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
Atoms & molecules
- 2. Laws of chemical combination
- 3. 1. Law of conservation of mass Law of conservation of mass states that mass can neither be created nor be destroyed.
- 4. 2. Law of constant proportions A pure chemical compound always contains same elements combined together in the same definite proportion by weight.
- 5. Postulates of Dalton’s atomic theory • All matter is composed of a very large number of very small particles called atoms. • Atoms are indivisible particles,which cannot be created or destroyed in a chemical reaction. • Atoms of a given element are identical in mass and chemical properties. • Atoms of different elements have different masses and chemical properties. • Atoms combine in the ratio of small whole numbers to form compounds. • The relative number and kinds of atoms are constant in a given compound.
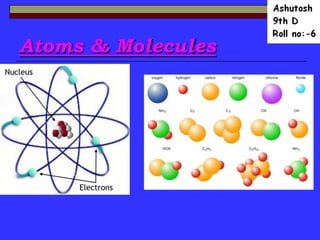
- 6. Atom • An atom is the smallest unit that maintains the charateristics of an element. -Nucleus- The center of the atom,contains protons and neutrons -Electron Cloud-Region surroundings the nucleus containing the electrons.
- 9. The Nucleus • Proton – A positively charged sub-atomic particle (+).The number of protons is the same as the atomic number. • Neutrons – A sub-atomic particle in the nucleus.Neutron do not have a charge(0).
- 10. Symbols of atoms of different elements
- 13. Molecules
- 14. Molecules • A molecule is defined as a stable neutral group of at least two atoms in a definite arrangement held together by very strong chemical bonds. • It can also be defined as a unit of two or more atoms held together by covalent bonds.
- 15. Chemical Bonds Covalent bonds form when atoms share 2 or more valence electrons. Covalent bond strength depends on the number of electron pairs shared by the atoms. single double < triple bond < bond bond
- 16. Chemical Bonds
- 17. Types of Bond
- 18. Covalent Bond