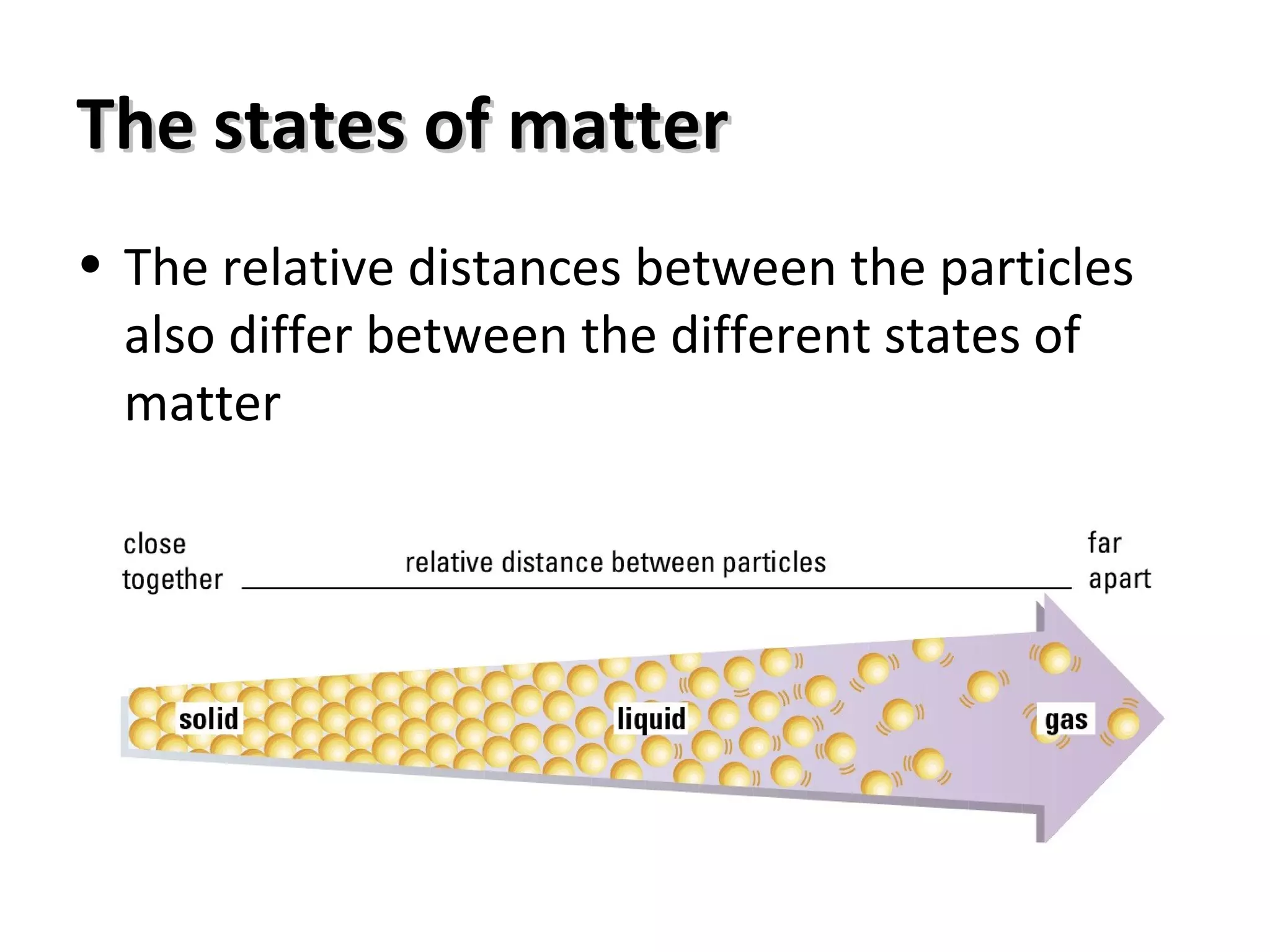
The document describes the key points of the kinetic particle theory. It explains that kinetic particle theory views matter as being made up of tiny particles in constant random motion. It also describes the three states of matter (solid, liquid, gas) in terms of the arrangement and motion of particles. Specifically, it explains how the properties of each state, such as compressibility and ability to change shape, can be understood by considering the forces between particles and how tightly or loosely packed they are.