Recommended
More Related Content
What's hot
What's hot (20)
PET - Quality Assurance of PET Radiopharmaceutials

PET - Quality Assurance of PET Radiopharmaceutials
FDA Form 483 (Inspectional Observations) - Top Violations 2013

FDA Form 483 (Inspectional Observations) - Top Violations 2013
Validation (intro, scope, merits, ich, who guidelines)

Validation (intro, scope, merits, ich, who guidelines)
Ruud Cox - Testing in a Medical Device Context - EuroSTAR 2012

Ruud Cox - Testing in a Medical Device Context - EuroSTAR 2012
How to Prepare for an FDA Inspection and Respond to FDA 483's / Warning Letters

How to Prepare for an FDA Inspection and Respond to FDA 483's / Warning Letters
InstantGMP Compliance Series - Managing Deviations for Improved Compliance

InstantGMP Compliance Series - Managing Deviations for Improved Compliance
Increase profit with process analytical technology eoswiss engineering slide...

Increase profit with process analytical technology eoswiss engineering slide...
Plan for Success Strategies to Align Reimbursement and Commercialization - OM...

Plan for Success Strategies to Align Reimbursement and Commercialization - OM...
Item 3. Internal quality control - Quality Control Charts

Item 3. Internal quality control - Quality Control Charts
Lab Inspections vs. Audits: What's the Difference & Why Does It Matter?

Lab Inspections vs. Audits: What's the Difference & Why Does It Matter?
Similar to DPL Compliance Road Map
Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROs

Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROsStatistics & Data Corporation
Incorporate CPV and Continual Improvement into your Validation Plan

Incorporate CPV and Continual Improvement into your Validation PlanInstitute of Validation Technology
Similar to DPL Compliance Road Map (20)
Understanding the Medical device Single Audit Program (MDSAP) & How to Prepar...

Understanding the Medical device Single Audit Program (MDSAP) & How to Prepar...
Computerized system validation (CSV) as a requirement for good manufacturing ...

Computerized system validation (CSV) as a requirement for good manufacturing ...
Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROs

Webinar: How to Ace Your SaaS-based EDC System Validation for Sponsors and CROs
PAT and QbD concepts in designing the LiMS and other Electronic systems in La...

PAT and QbD concepts in designing the LiMS and other Electronic systems in La...
Incorporate CPV and Continual Improvement into your Validation Plan

Incorporate CPV and Continual Improvement into your Validation Plan
Facility Qualification & Consideration of Validation Aspects 

Facility Qualification & Consideration of Validation Aspects
Effective Medical Device Validation Introduction Web 2

Effective Medical Device Validation Introduction Web 2
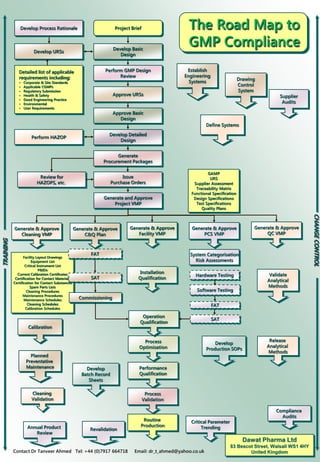
DPL Compliance Road Map
- 1. Critical Parameter Trending Issue Purchase Orders The Road Map to GMP Compliance TRAINING CHANGECONTROL Dawat Pharma Ltd 83 Bescot Street, Walsall WS1 4HY United KingdomContact Dr Tanveer Ahmed Tel: +44 (0)7917 664718 Email: dr_t_ahmed@yahoo.co.uk Develop URSs Develop Basic Design Develop Process Rationale Project Brief Define Systems Develop Production SOPs Drawing Control System Supplier Audits Approve URSs Approve Basic Design Perform GMP Design Review Generate Procurement Packages Develop Detailed Design Detailed list of applicable requirements including: • Corporate & Site Standards • Applicable CGMPs • Regulatory Submission • Health & Safety • Good Engineering Practice • Environmental • User Requirements Perform HAZOP Review for HAZOPS, etc. Generate and Approve Project VMP Develop Batch Record Sheets Release Analytical Methods Compliance Audits Cleaning Validation Annual Product Review Validate Analytical Methods Establish Engineering Systems Facility Layout Drawings Equipment List Critical Instrument List P&IDs Current Calibration Certificates Certification for Contact Materials Certification for Contact Substances Spare Parts Lists Cleaning Procedures Maintenance Procedures Maintenance Schedules Cleaning Schedules Calibration Schedules System Categorisation Risk Assessments FAT SAT Software Testing Hardware Testing Generate & Approve QC VMP Generate & Approve PCS VMP Generate & Approve Facility VMP Generate & Approve Cleaning VMP Generate & Approve C&Q Plan Process Optimisation Operation Qualification Installation Qualification Performance Qualification Process Validation Routine Production GAMP URS Supplier Assessment Traceability Matrix Functional Specification Design Specifications Test Specifications Quality Plans SAT Commissioning FAT Revalidation Calibration Planned Preventative Maintenance