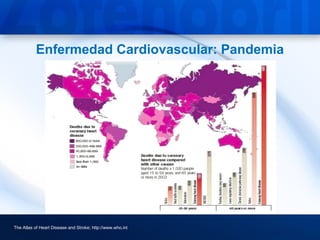
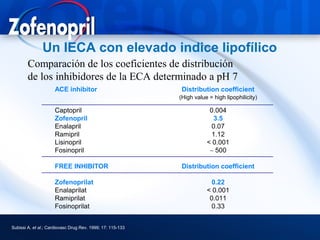
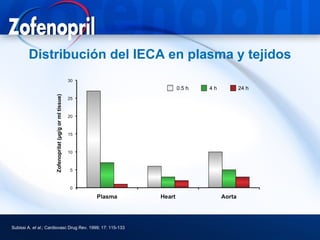
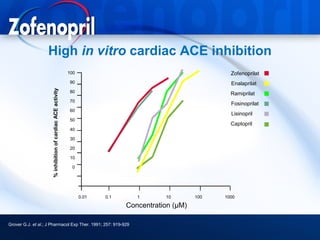
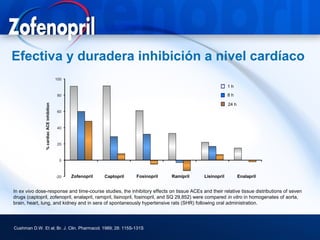
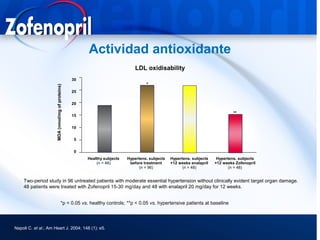
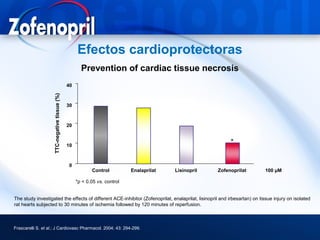
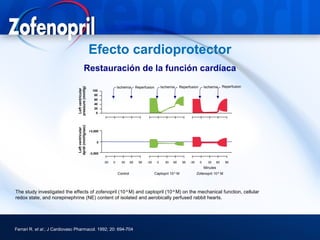
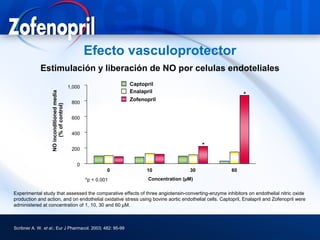
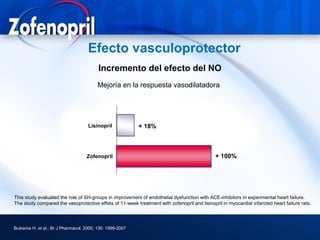
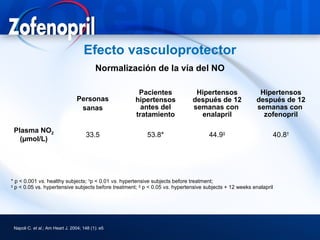
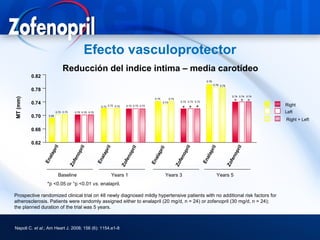
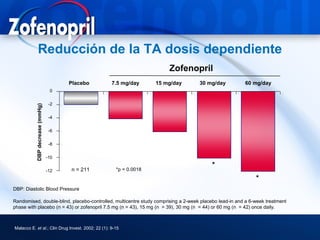
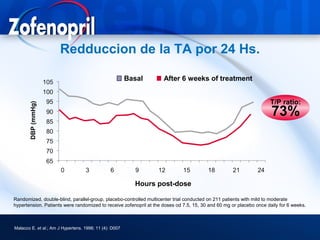
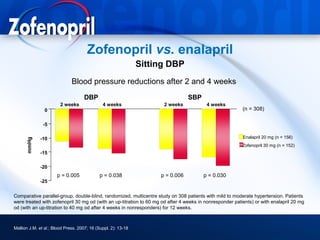
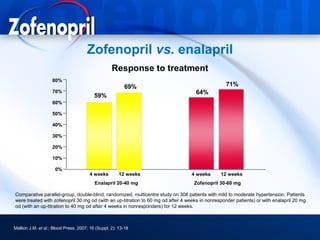
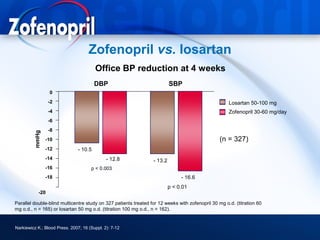
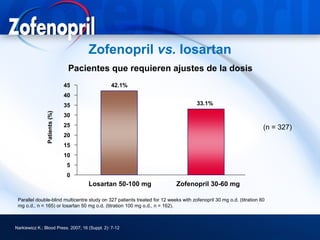
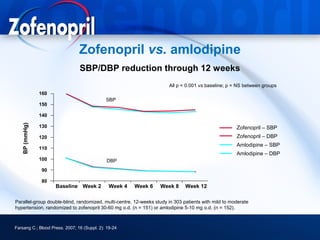
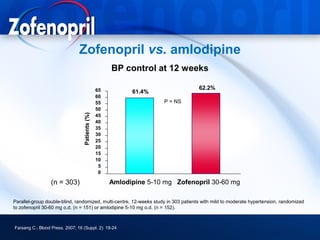
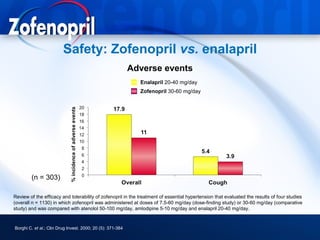
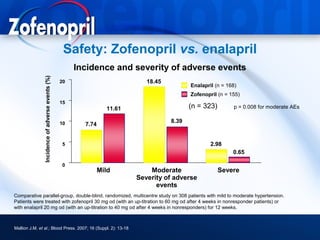
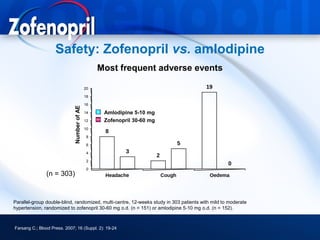
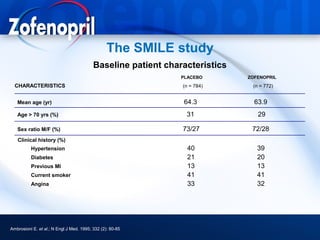
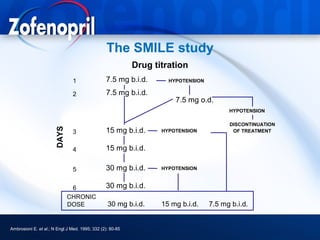
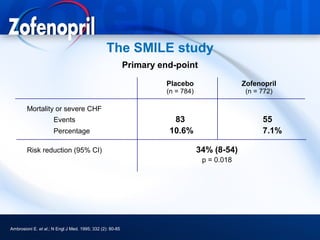
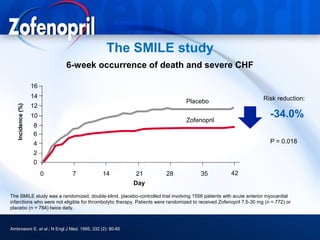
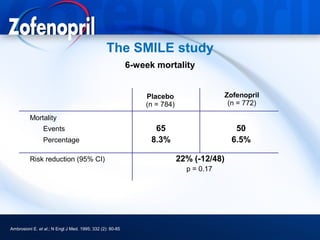
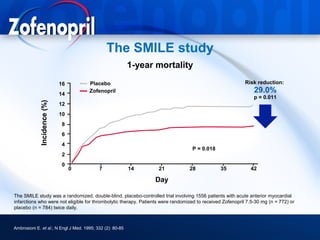
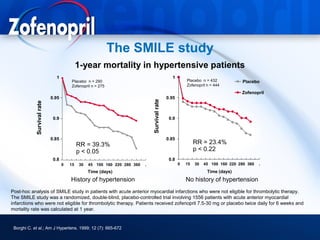
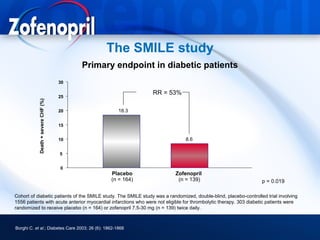
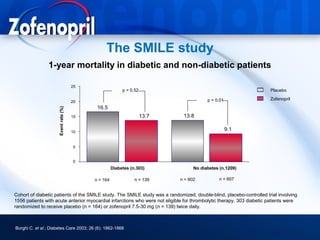
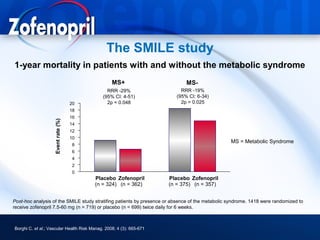
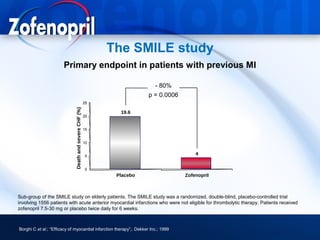
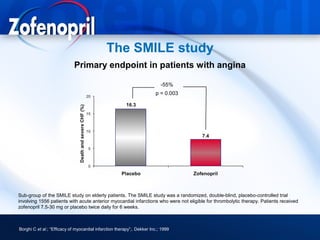
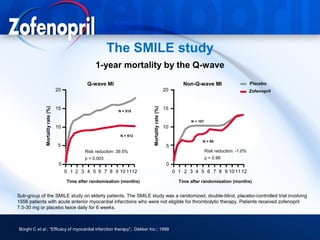
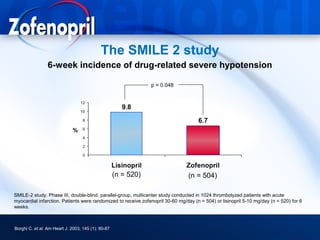
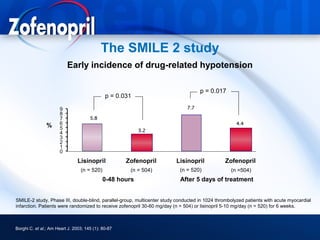
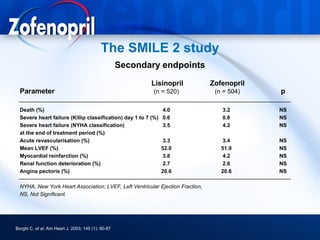
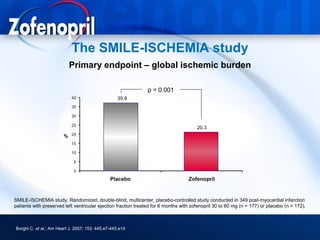
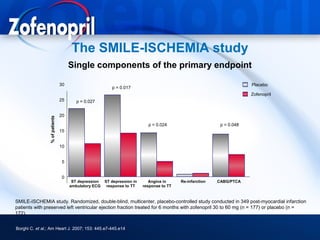
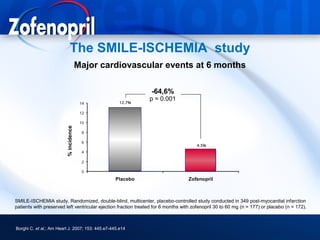
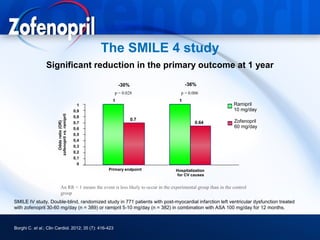
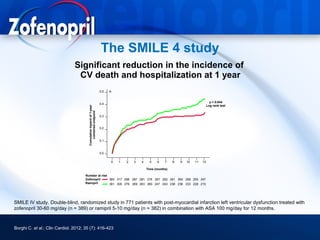
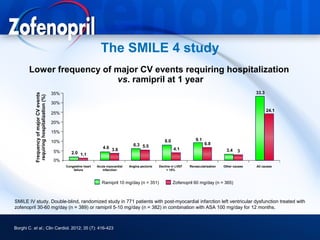
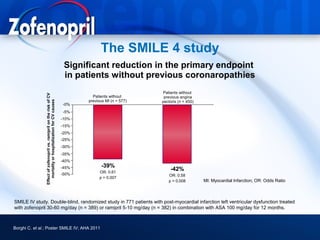
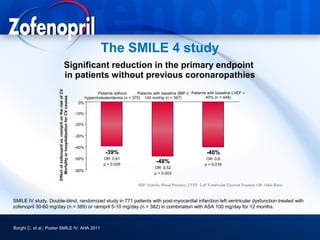
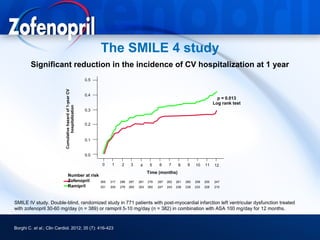
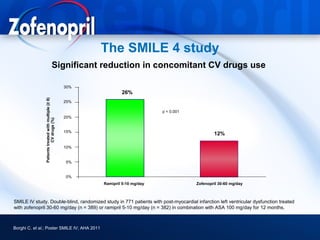
This document discusses hypertension and cardiovascular disease, and compares guidelines for treating hypertension from JNC7 and ESH/ESC. It provides the following key points:
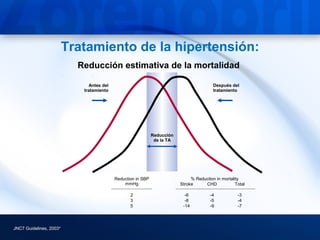
1) Treatment of hypertension can significantly reduce mortality from stroke and coronary heart disease. Reductions in blood pressure of just 2-5 mmHg can lower mortality risks.
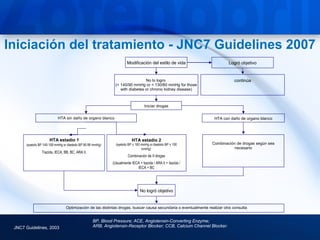
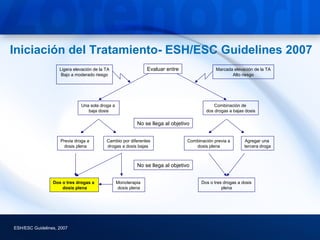
2) Guidelines recommend treating hypertension to target blood pressures of less than 140/90 mmHg, and less than 130/80 mmHg for those with diabetes or chronic kidney disease.
3) Angiotensin converting enzyme (ACE) inhibitors play a major role in hypertension treatment, both as monotherapy and in combination with other drugs like diuretics and calcium channel block