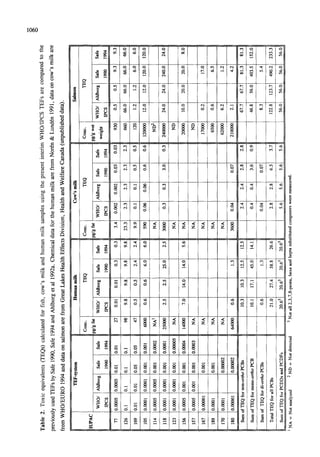
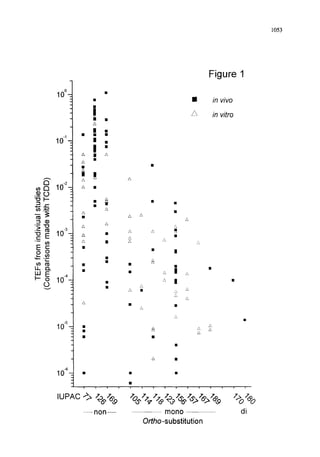
This document summarizes a consultation held by the WHO-ECEH and IPCS to derive toxic equivalency factors (TEFs) for dioxin-like polychlorinated biphenyls (PCBs). Researchers collected data on the relative toxicities of PCBs compared to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) from studies. At the consultation, they analyzed the data to define criteria for TEFs and derive TEFs for 13 PCBs. TEFs indicate the relative toxicity of PCBs compared to TCDD. The consultation recommended expanding the project to include more compounds and exploring separate TEFs for different applications like




![1054
e9
o° m
1=:
O
J3
E
Z
30
20
10
Figure 2
A
60t
50 I--]
B
Comparison with TCDD
Comparison with PCB 126
Comparison with PCB 77 or PCB 169
40
¢--
o 30
E
z 20
10-
O ~
lUPAC -~.~%%
--non-- mono di
Ortho-substitution](https://image.slidesharecdn.com/yzd542-150101010200-conversion-gate02/85/Toxic-Equivalency-Factors-for-Dioxin-Like-PCBs-6-320.jpg)