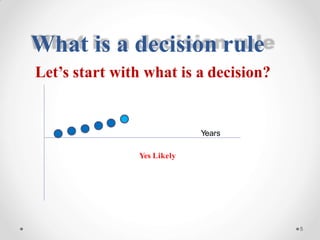
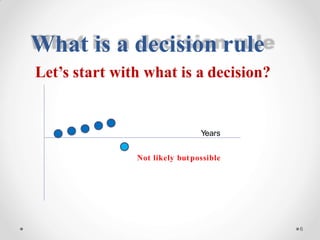
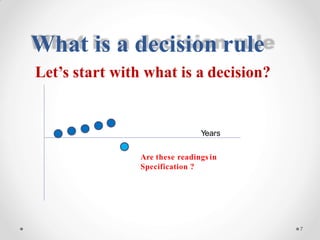
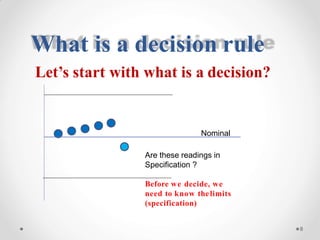
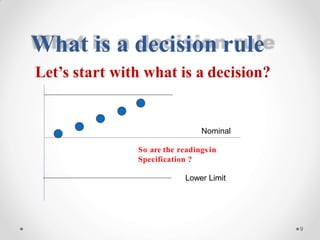
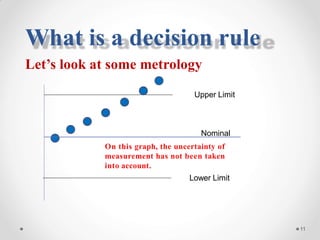
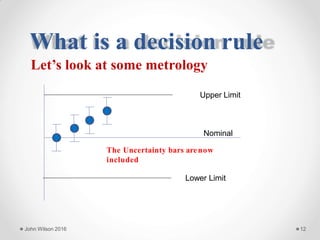
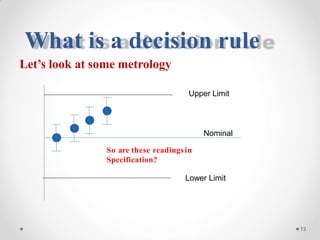
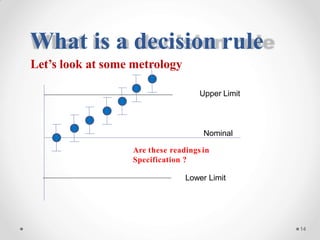
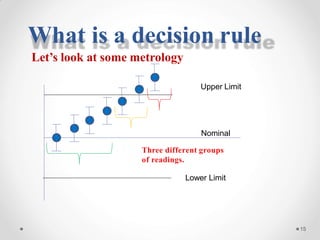
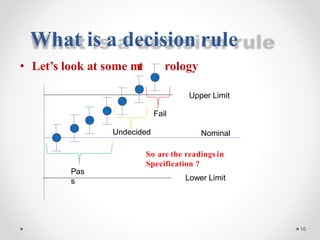
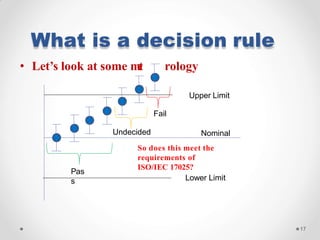
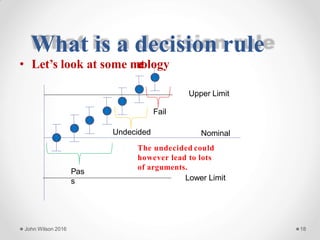
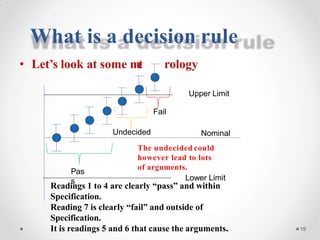
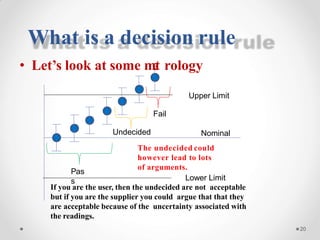
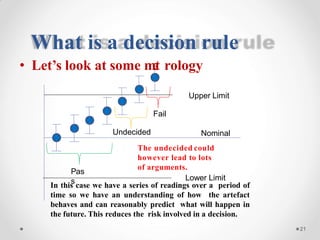
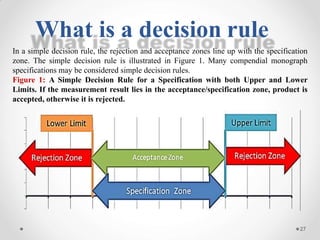
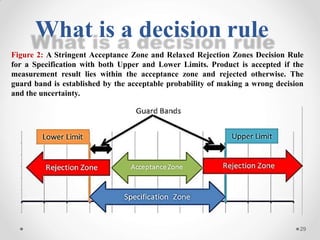
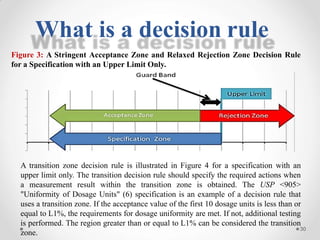
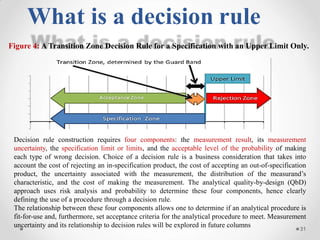
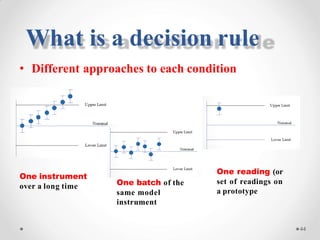
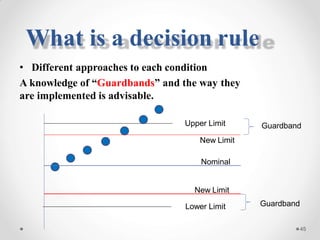
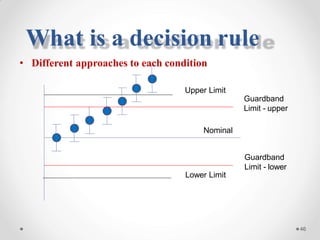
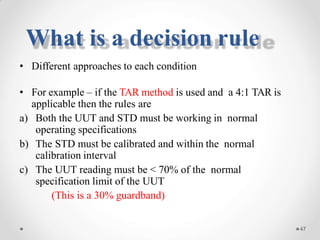
The document discusses the concept of decision rules in the context of ISO/IEC 17025:2005 and 2017, emphasizing the importance of incorporating measurement uncertainty when making statements of conformance. It outlines various decision-making scenarios, the implications of false accept and reject risks, and the necessity for clear documentation and communication between laboratories and customers regarding compliance criteria. Additionally, it highlights the need for laboratories to understand different decision rule models depending on their testing conditions and to provide adequate detail in reports to ensure consistent interpretations of results.