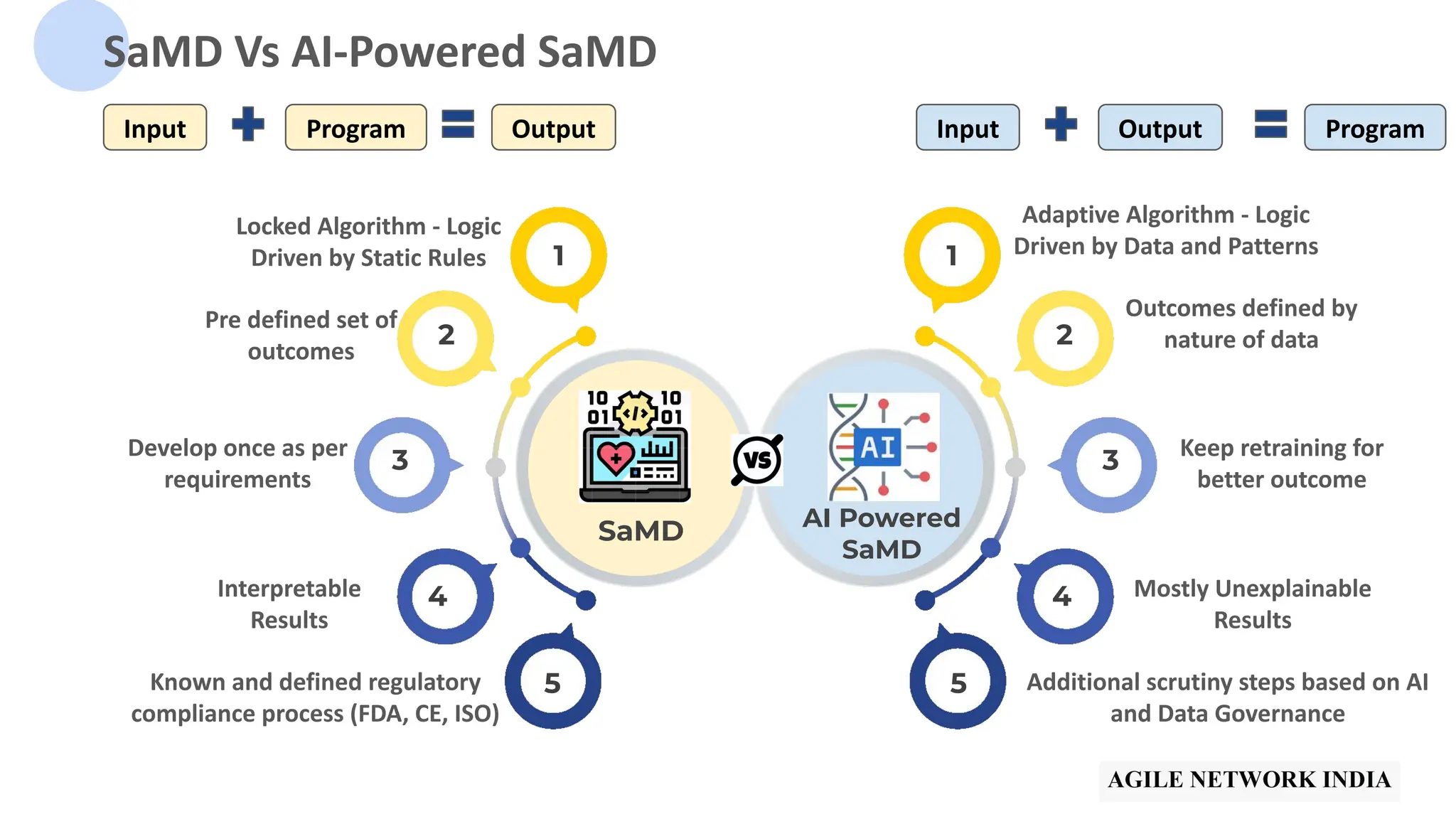
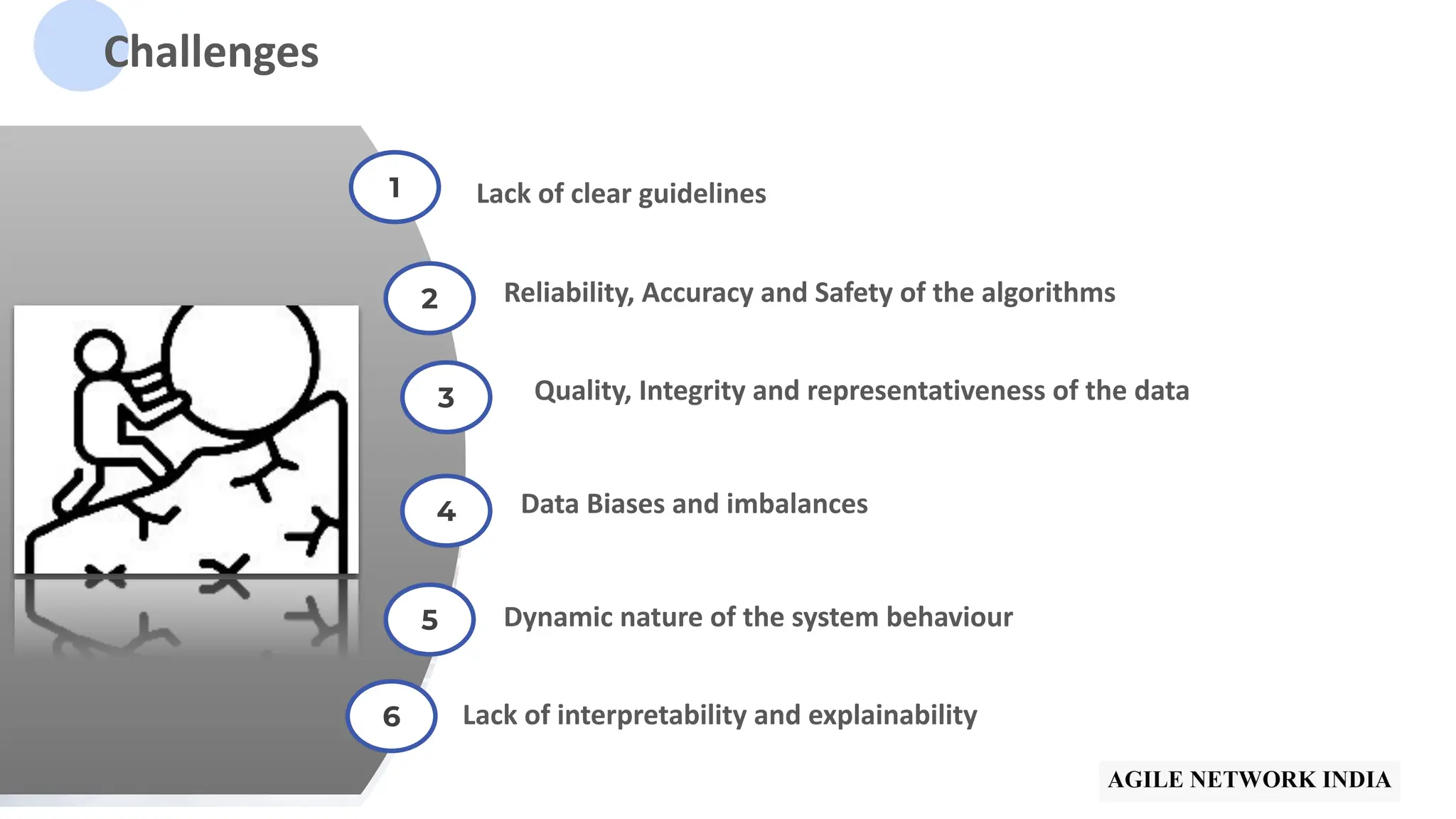
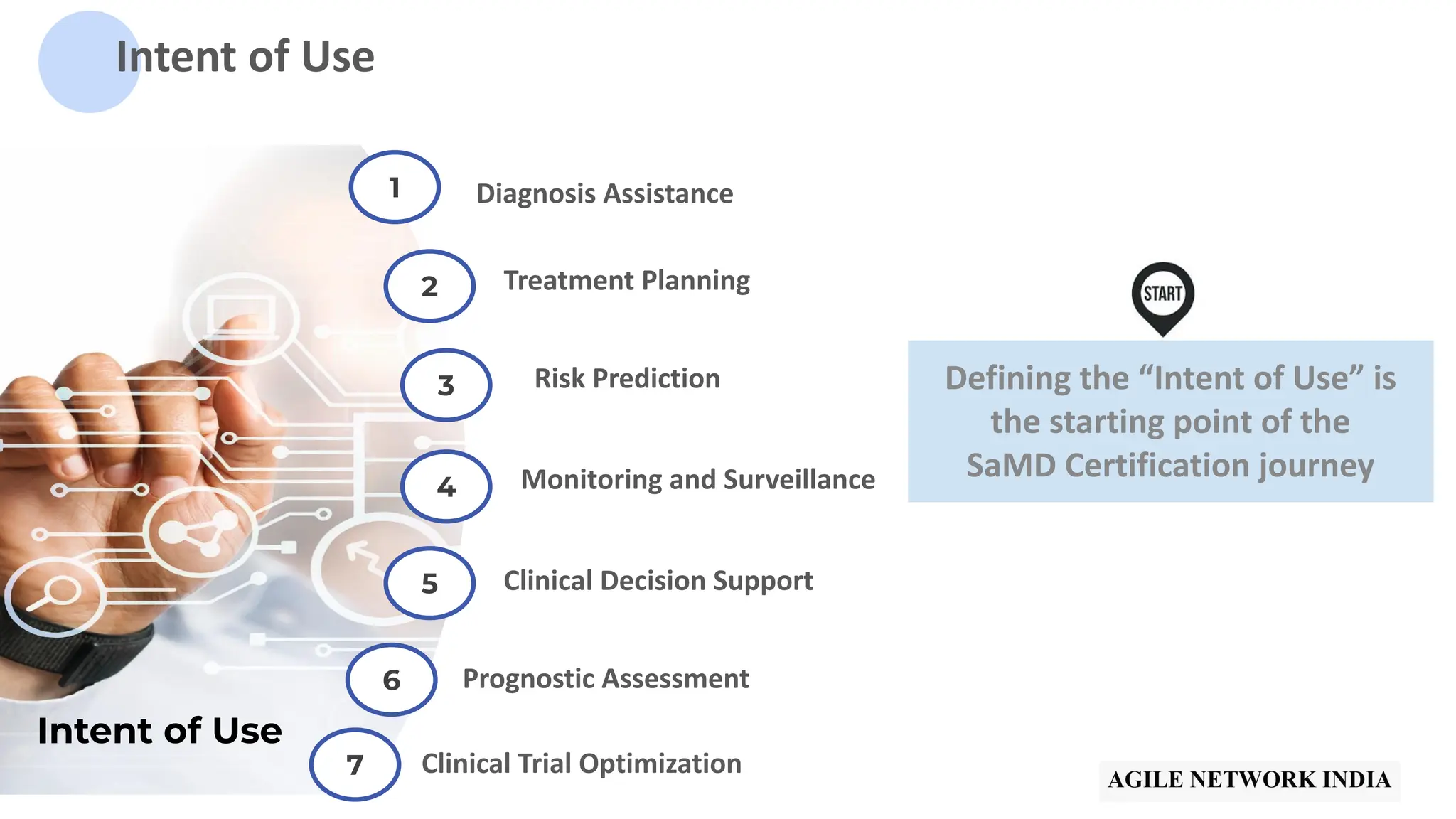
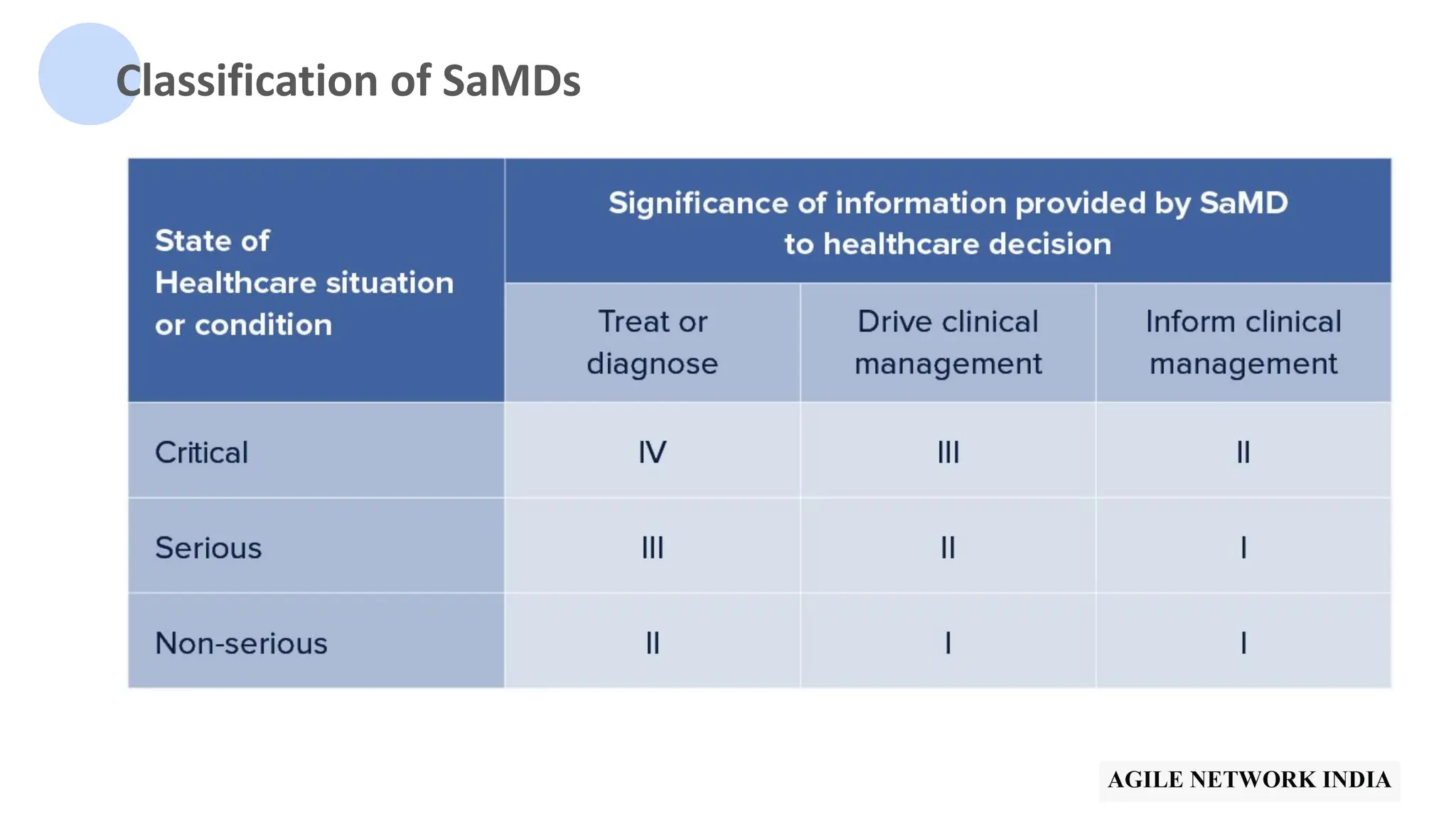
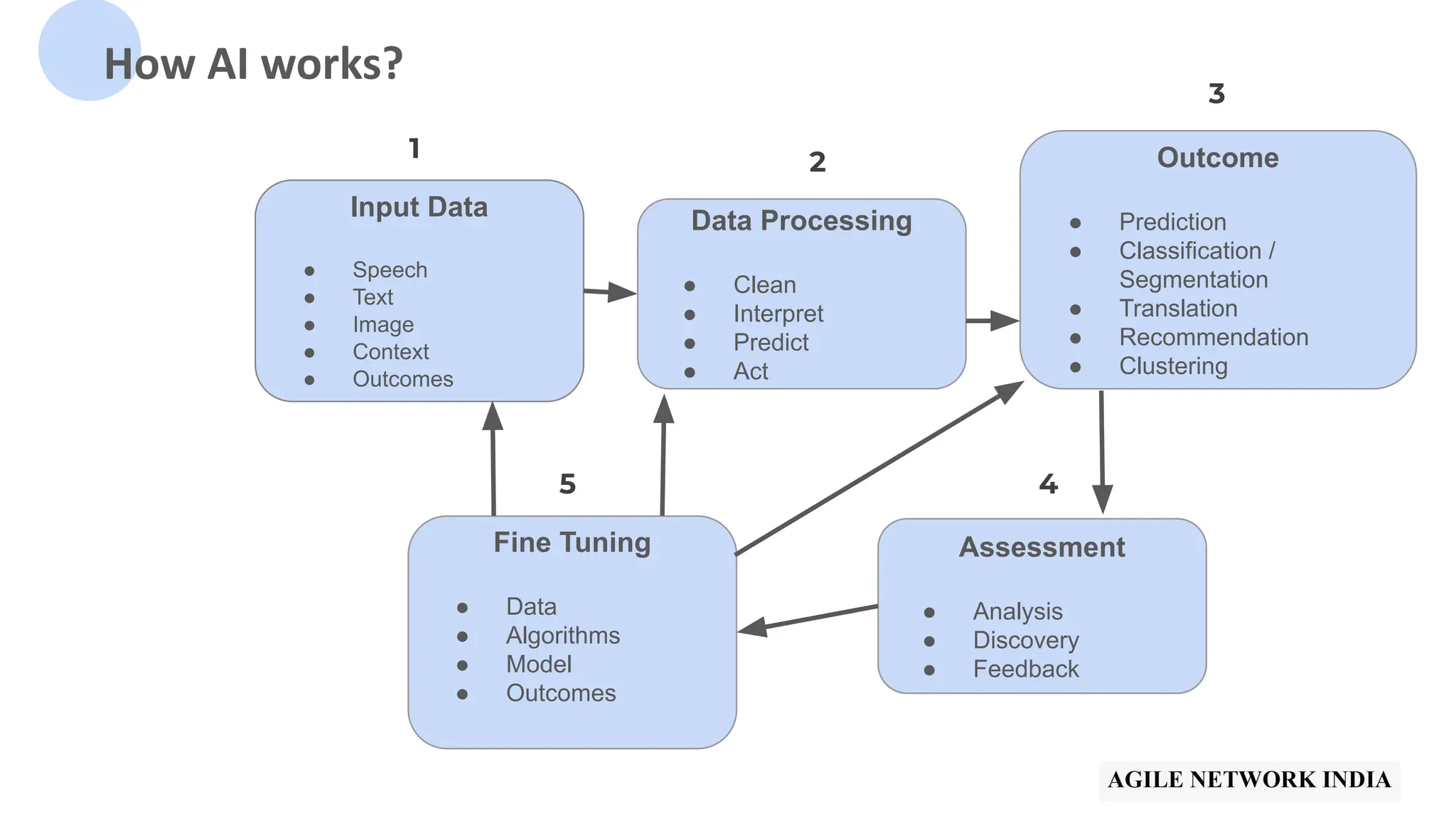
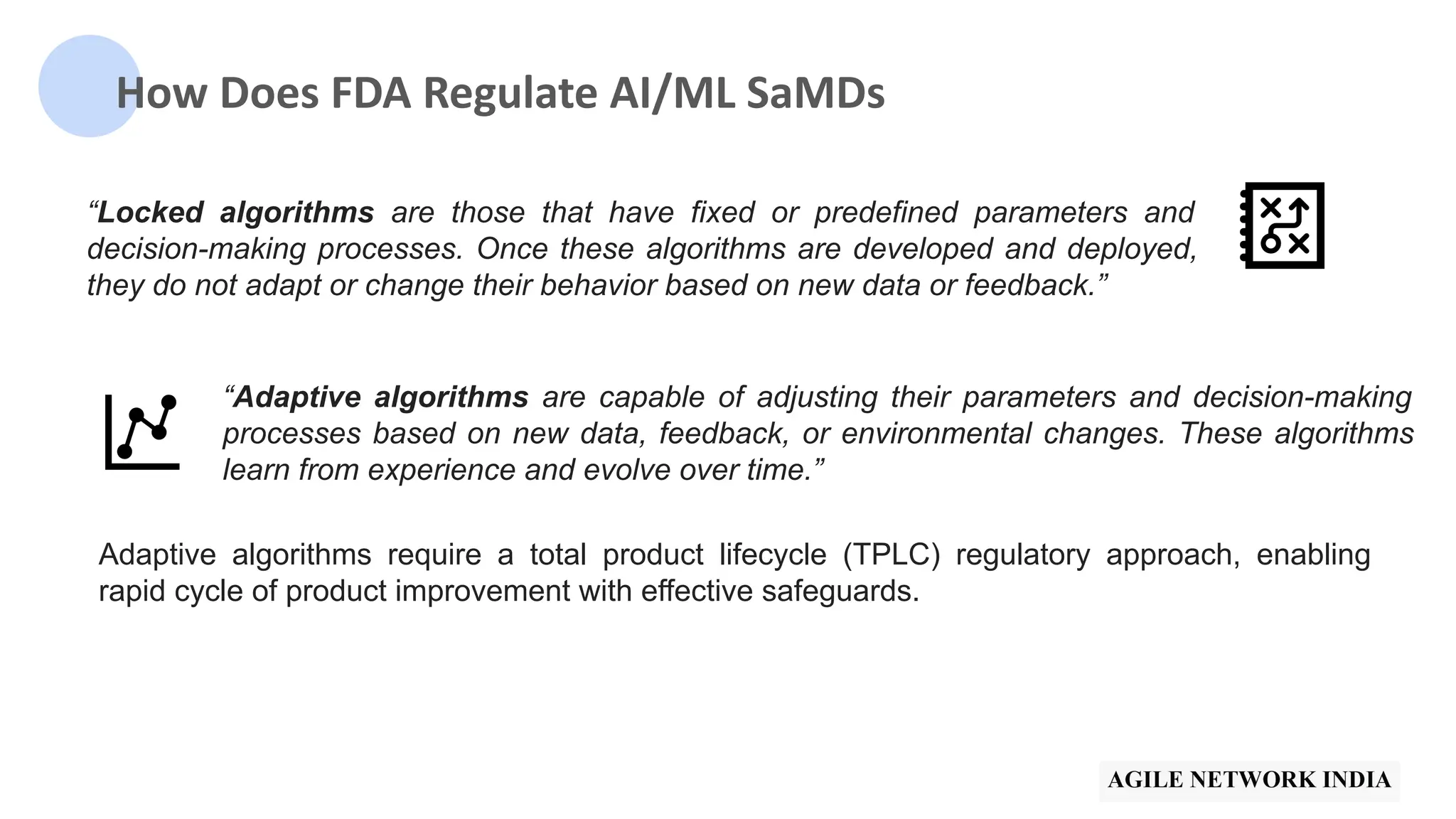
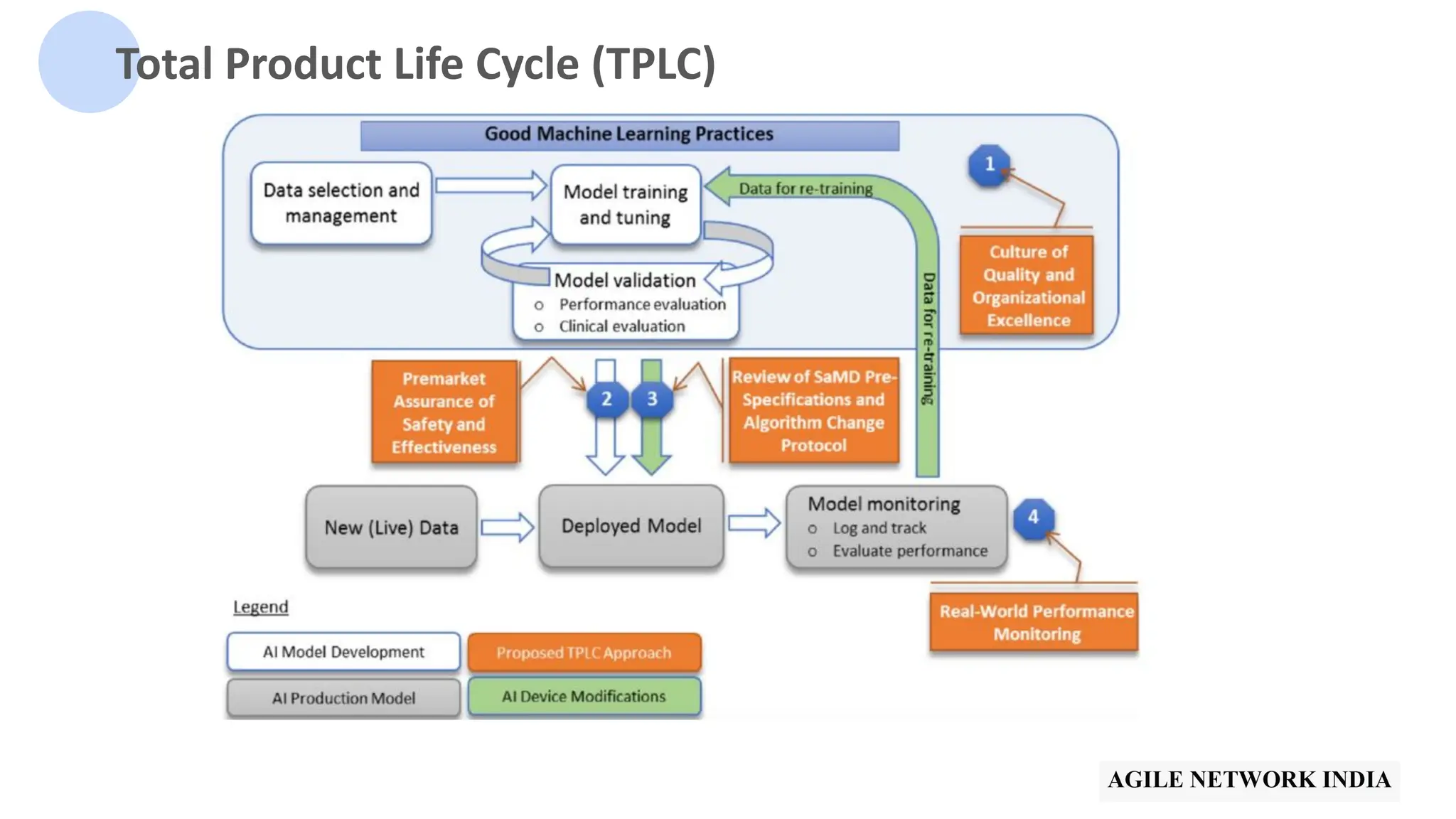
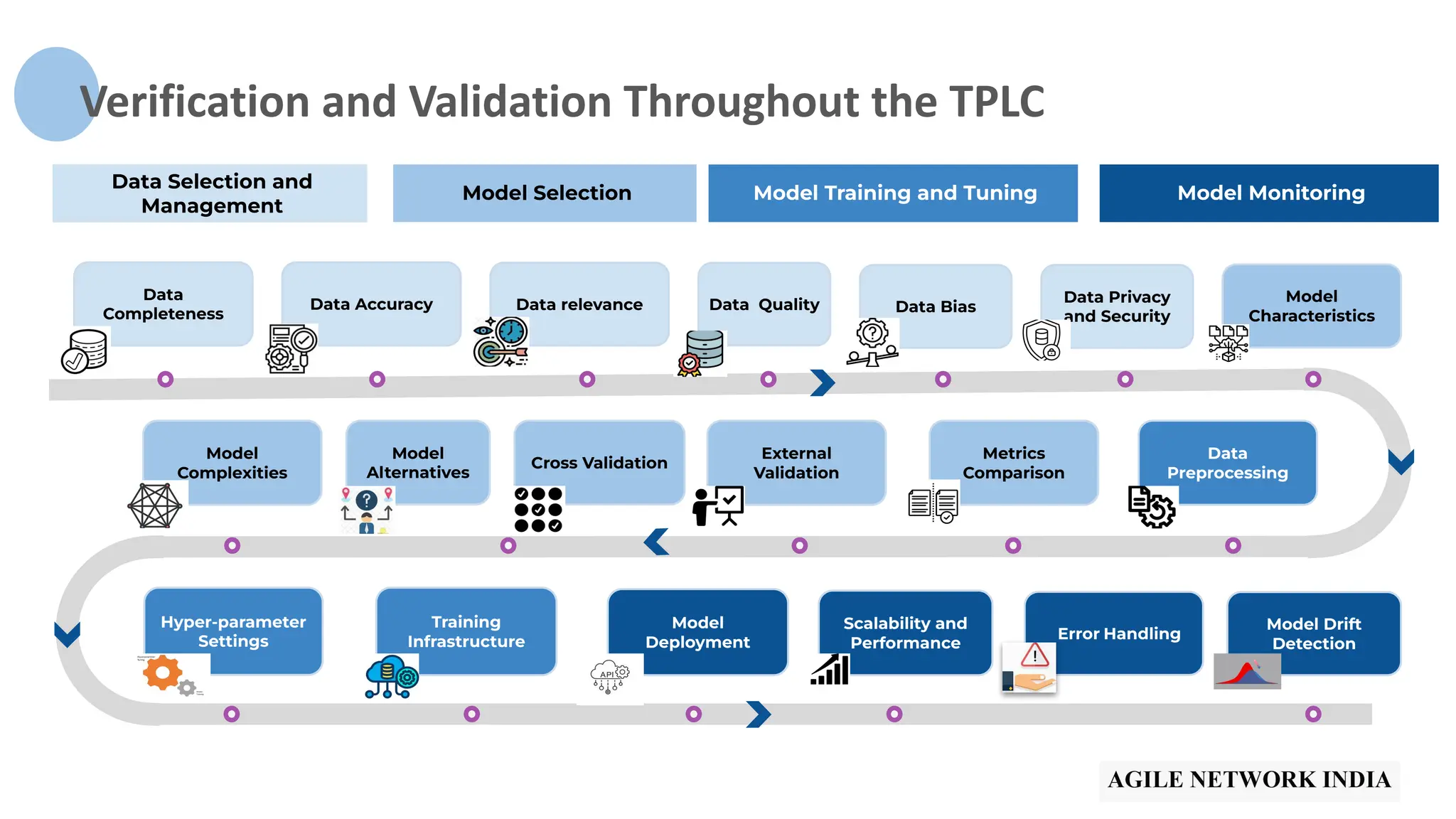
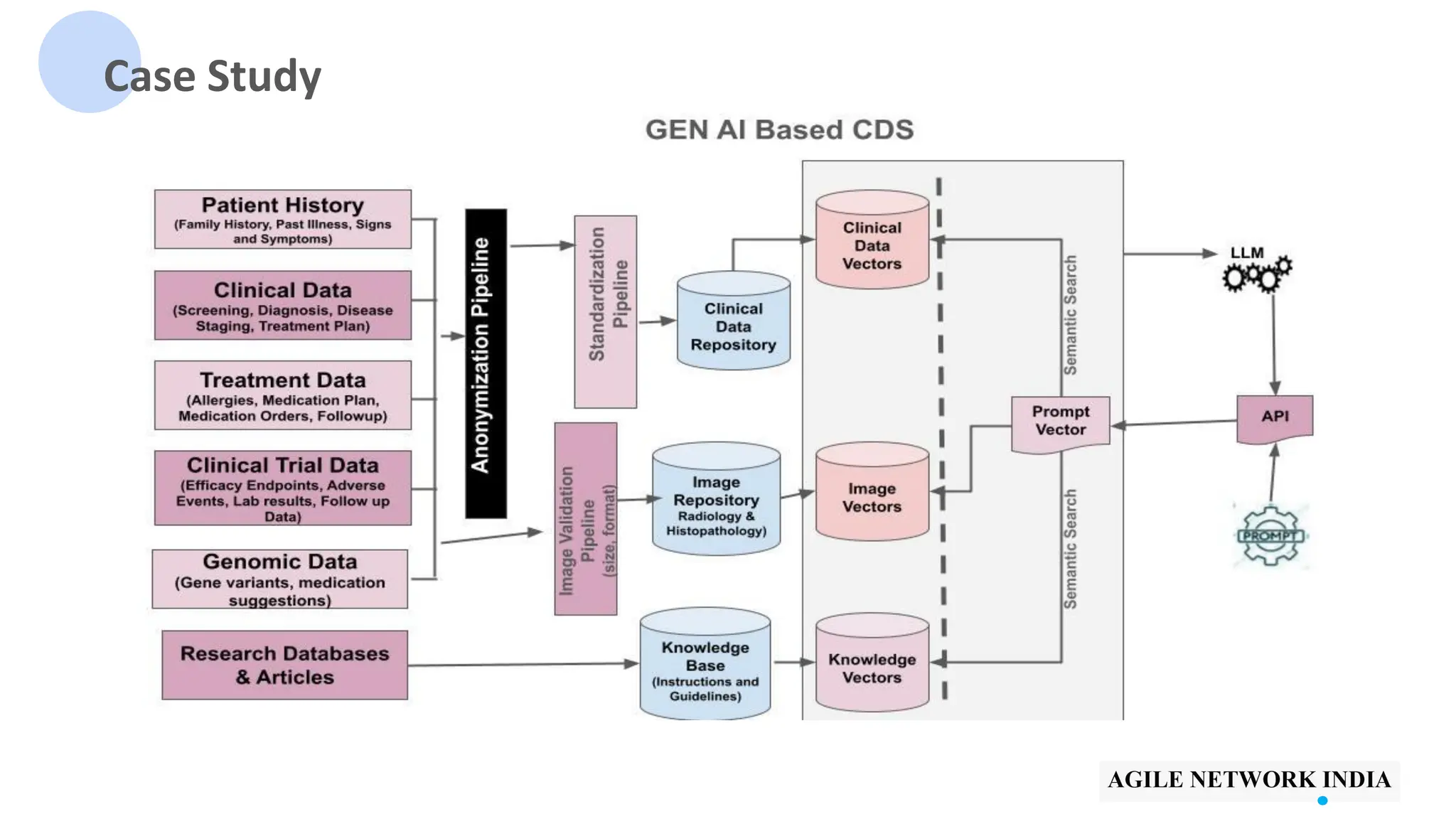
The presentation discusses best practices in testing Software as a Medical Device (SaMD) powered by AI, highlighting challenges in obtaining certification, regulatory frameworks, and key concepts in quality management systems for these devices. It distinguishes between locked and adaptive algorithms and emphasizes the importance of regulatory compliance, data integrity, and continuous improvement. A case study is included to illustrate the implementation of AI-powered SaMD.




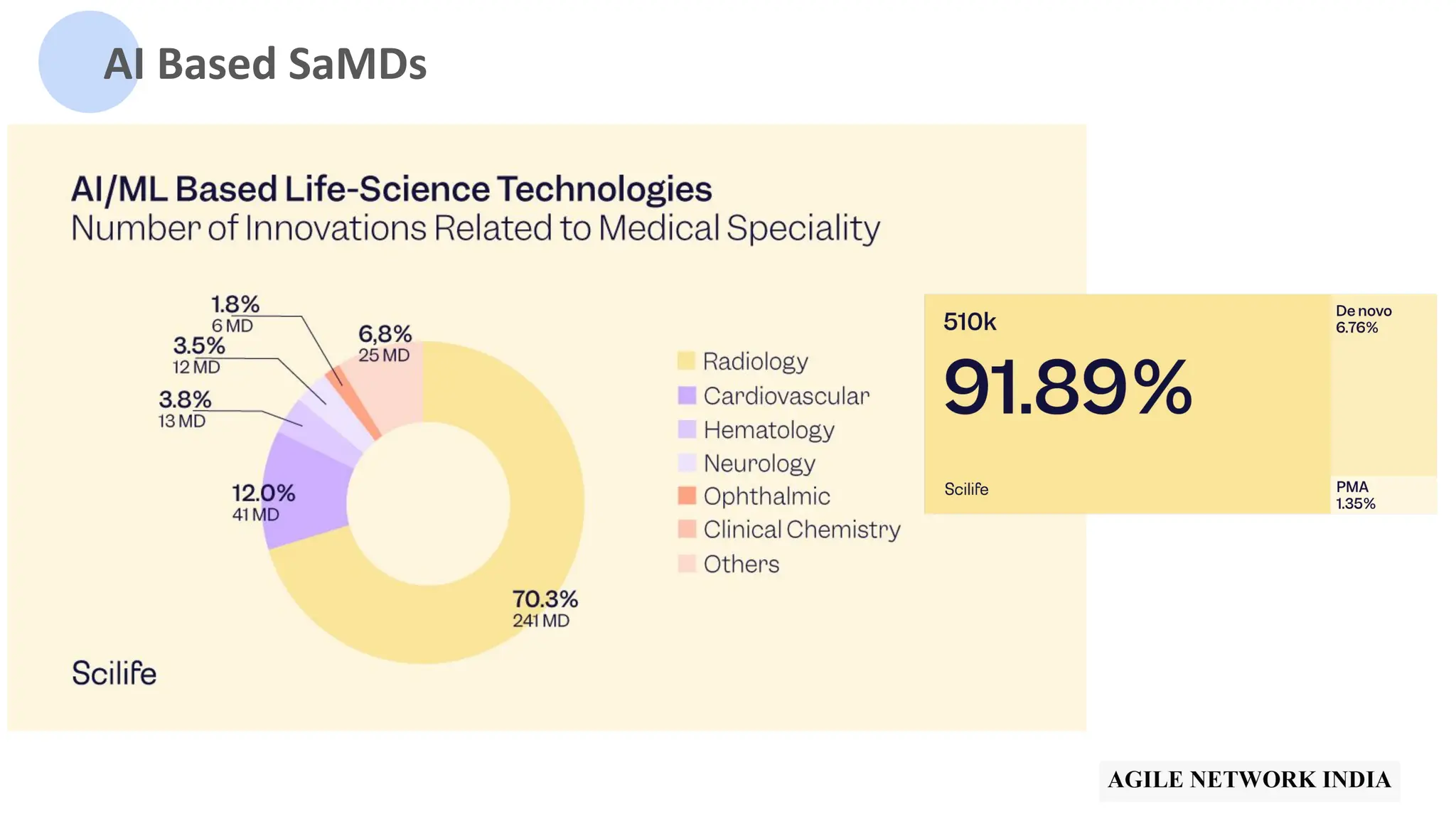
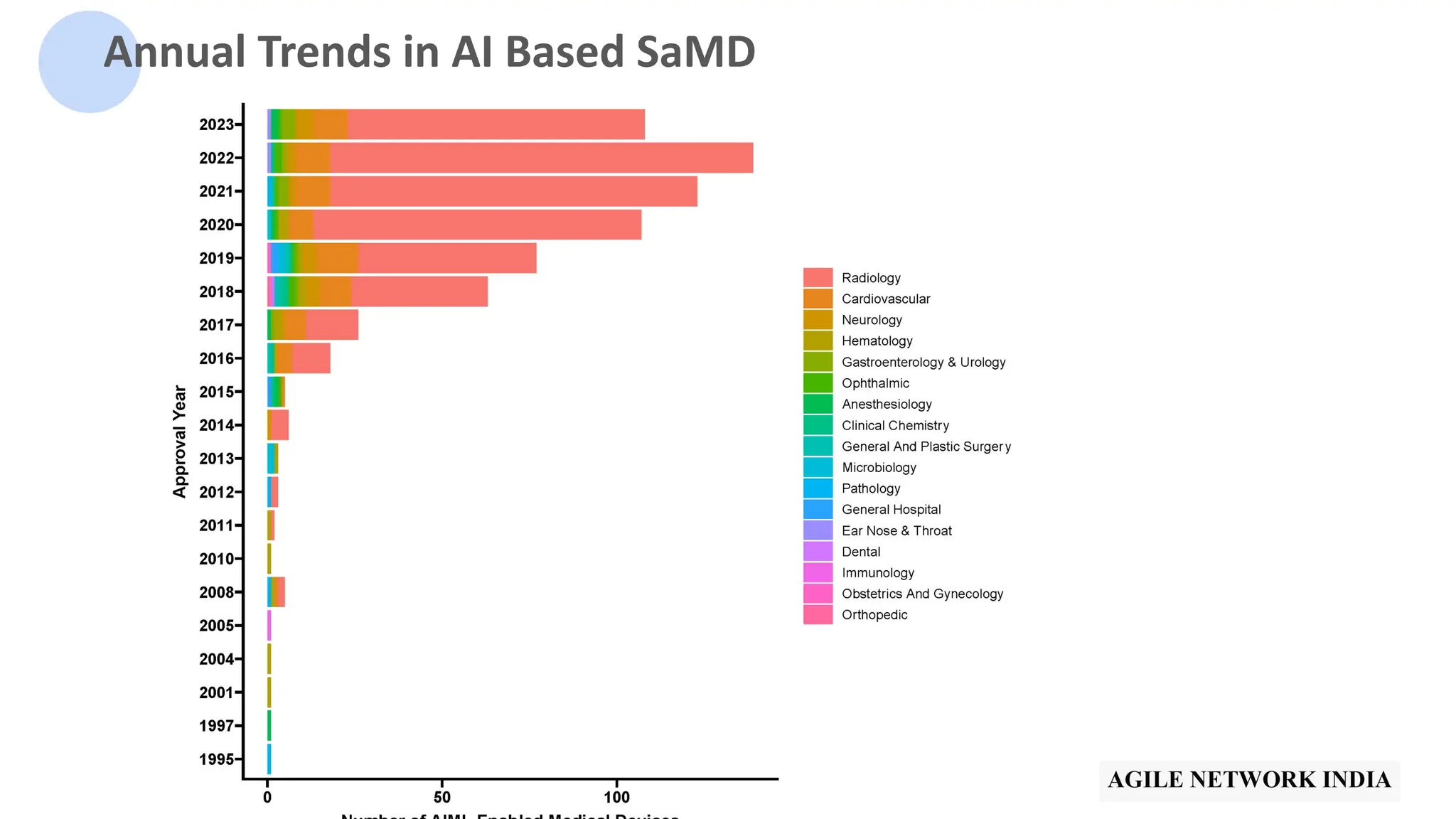
![510(k) Vs PMA Vs De Novo
[Image from IMARC]](https://image.slidesharecdn.com/bestpracticesintestingaipoweredsamdv1-240513041528-cf3a7d7f/75/ANIn-Pune-May-2024-Best-practices-in-testing-of-AI-based-SaMD-by-Anupama-Ananthasairam-11-2048.jpg)
![References
[1] Brady, Adrian & Allen, Bibb & Chong, Jaron & Kotter, Elmar & Kottler, Nina & Mongan, John & Oakden-Rayner, Lauren & Pinto dos
Santos, Daniel & Tang, An & Wald, Christoph & Slavotinek, John. (2024). Developing, purchasing, implementing and monitoring AI
tools in radiology: practical considerations. A multi-society statement from the ACR, CAR, ESR, RANZCR & RSNA. Insights into imaging.
15. 16. 10.1186/s13244-023-01541-3.
[2] Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a
Medical Device (SaMD) -Discussion Paper and Request for Feedback. (n.d.). Available at:
https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf.
[3] Gilbert S, Fenech M, Hirsch M, Upadhyay S, Biasiucci A, Starlinger J. Algorithm Change Protocols in the Regulation of Adaptive
Machine Learning-Based Medical Devices. J Med Internet Res. 2021 Oct 26;23(10):e30545. doi: 10.2196/30545. PMID: 34697010;
PMCID: PMC8579211.
[4] www.scilife.io. (n.d.). FDA’s Regulatory Framework for AI/ML Technologies | Scilife. [online] Available at:
https://www.scilife.io/blog/fda-regulatory-framework-ai-ml [Accessed 6 May 2024].
[5] MATRIX, O.S.A. (2018). What is a Medical Device Quality Management System (QMS)? [online] Oriel STAT A MATRIX Blog. Available
at: https://www.orielstat.com/blog/medical-device-qms-overview/.](https://image.slidesharecdn.com/bestpracticesintestingaipoweredsamdv1-240513041528-cf3a7d7f/75/ANIn-Pune-May-2024-Best-practices-in-testing-of-AI-based-SaMD-by-Anupama-Ananthasairam-23-2048.jpg)
![References (cont)
[6] Joshi, G., Jain, A., Shalini Reddy Araveeti, Adhikari, S., Garg, H. and Bhandari, M. (2024). FDA-Approved Artificial Intelligence and
Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape. Electronics, 13(3), pp.498–498.
doi:https://doi.org/10.3390/electronics13030498.](https://image.slidesharecdn.com/bestpracticesintestingaipoweredsamdv1-240513041528-cf3a7d7f/75/ANIn-Pune-May-2024-Best-practices-in-testing-of-AI-based-SaMD-by-Anupama-Ananthasairam-24-2048.jpg)