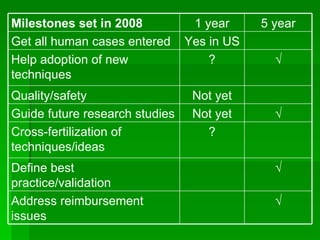
The 4th International Conference on NOTES Working Group 1 discussed the development of a registry to track natural orifice transluminal endoscopic surgery (NOTES) and hybrid cases. The registry currently includes 166 cases from a few US sites. Goals are to provide more safety and complication data to regulatory agencies and help adoption of new techniques. Milestones in the next 1-5 years include increasing US participation to 100% and helping to define best practices through data analysis. Ways to improve the registry include enhancing international cooperation and data sharing, and incentivizing participation.